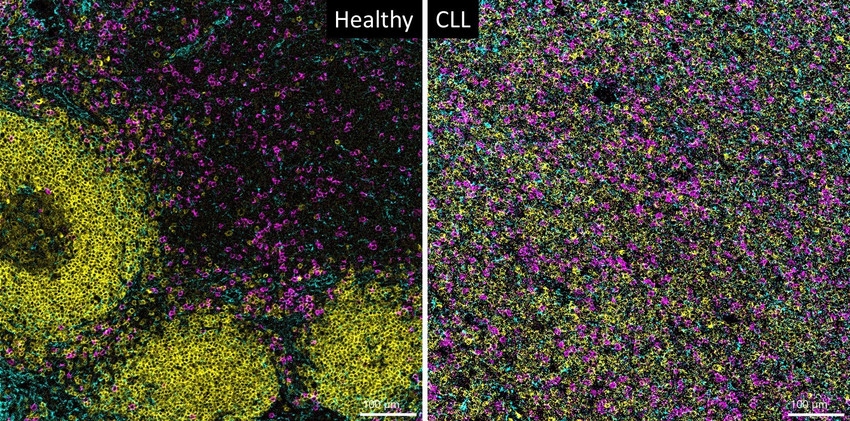
Treatment for B cell malignancies has shifted towards a chemo-free regiment with the combination of targeted therapy and/or immunotherapy. However, resistance to these therapeutics has emerged and new complementary options are still urgently needed for these cases. Regulation of T cell activity and restoration of T cell-mediated anti-tumor responses represent a prime research focus for immunotherapy. However, tumor cells can employ various mechanisms to evade anti-tumor immune responses, leading to the failure of cytotoxic T cells to efficiently proliferate and form immunologic synapses. The deregulated expression of diverse immune checkpoint molecules as well as co-stimulatory receptors have been described to modulate the activation of major T cell subsets. Moreover, the modulating effect of stromal cells on lymphoma development and T cell activity has been increasingly acknowledged. Stromal cells not only contribute to tumor growth via the interdependent generation of a nurturing tumor niche, but also adopt a number of other immunosuppressive mechanisms to modulate immune responses resulting in an enhanced immune tolerance to the tumor.
The main goal of this project is to understand the complex regulation of T cell immunity by both the stroma and lymphoma cells to develop new therapeutic combinations. Particularly, regimens combining targeted- and immunotherapies will be tested in different in vitro and in vivo models.
The modulation of anti-lymphoma T cell activity is not fully elucidated. Understanding the multifaceted regulation of T cell responses by both malignant cells and the stromal microenvironment will provide more insights into the effectiveness as well as vulnerability of T cell-based immunotherapy for B cell lymphoma. Ultimately, our project seek to develop preclinically complementary therapies against lymphoma hindering immune-suppressions caused by stromal deregulations and T cell exhaustion.

Clinic I for Internal Medicine
CMMC - PI - A 09
CMMC - PI - CAP 19
+49 221 478 84120
Clinic I for Internal Medicine
Joseph-Stelzmann Str. 26
50931 Cologne

Institute of Translational Immune-Oncology - Clinic I of Internal Medicine - Chair of Translational Immune-Oncology
CMMC - Co-PI - A 09
renata.stripecke[at]uk-koeln.de
show more…+49 221 478 51457
Institute of Translational Immune-Oncology - Clinic I of Internal Medicine - Chair of Translational Immune-Oncology
Weyertal 115c
50931 Cologne
https://innere1.uk-koeln.de/forschung/arbeitsgruppen-labore/translational-immune-oncology/

Center for Molecular Medicine Cologne | Lab. of Cell Death, Inflammation and Immunity - CMMC Research Building
CMMC - Co-PI - A 09
CMMC - PI - JRG 12
alessandro.annibaldi[at]uk-koeln.de
show more…+49 221 478 37455
Center for Molecular Medicine Cologne | Lab. of Cell Death, Inflammation and Immunity - CMMC Research Building
Robert-Koch-Str. 21
50931 Cologne
PhD students:
Liudmila Lobastova
Thanh-Tung Truong
Viktoria Kohlhas
Technician:
H. Bohner