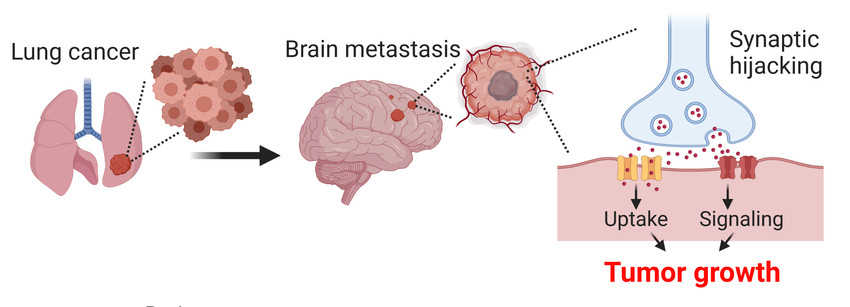
The finding that intractable, malignant brain tumors (i.e., glioma and glioblastoma) can hijack the local synaptic environment to sustain their own growth and invasiveness has recently sparked much attention. These findings uncovered a previously unknown vulnerability in intractable brain tumors, where synaptic terminals are most abundant, yet the key question arises whether other types of malignant tumors outside the brain may also utilize similar mechanisms to maintain proliferation in situ or even to disseminate into brain tissue. Building on preliminary data, we focus here on small lung cancer cells (SCLC) to investigate their capability to receive synaptic inputs by neurons and explore the possibility that this process may be relevant for SCLC brain metastasis formation and growth – a hallmark of poor survival in patients.
SCLC is a highly metastatic neuroendocrine carcinoma with poor response to chemotherapy or radiation. SCLC dissemination to the brain is a major cause of mortality in patients, underscoring the need to identify new molecular targets for effective treatments. The planned experiments are meant to unambiguously identify the relevance of synaptic hijacking for SCLC metastatic growth and lay the ground for the translational applicability of our findings for new effective therapies.
By employing state-of-the-art synaptic tracing, chemogenetics and imaging approaches, we will investigate to which extent the growing ability of SCLC cells may rely upon synaptic hijacking (Figure 1). By these experiments we hope to acquire key insights into the mechanisms promoting SCLC brain metastatic growth.
Specific approaches:
1) Establishment of neuron-cancer cell co-cultures to investigate changes in proliferation and neurotoxicity
2) Trans-synaptic tracing approach to map the potential connectome of cancer cells
3) Viral-based approaches to manipulate gene expression in cancer cells and neurons

CECAD Cologne
CMMC - PI - A 02
show more…+49 221 478 84250
+49 221 478 78910
CECAD Cologne
Joseph-Stelzmann-Str. 26
50931 Cologne
CECAD: https://www.cecad.uni-koeln.de/research/principal-investigators/prof-dr-matteo-bergami/
CRC1218: https://sfb1218.uni-koeln.de/projects/research-area-a-mitochondrial-dynamics-and-quality-control/a07-bergami
CRC1451: https://www.crc1451.uni-koeln.de/index.php/a03/

Institute of Biochemistry
CMMC - Co-PI - A 02
show more…+49 221 470 76056
Institute of Biochemistry
Zülpicher Str. 47
50674 Cologne
https://sfb1218.uni-koeln.de/projects/research-group-leaders/motori-lab
PostDoc
Kristiano Ndoci, Hannah Jahn, Milica Jevtic
PhD student
Abdulla Chihab, Marco Niestroj
Master student
Ann-Sophie Benz