Pancreatic ductal adenocarcinoma (PDAC) is amongst the deadliest cancers with a 5-year survival rate of below 5%. This poor prognosis is caused by absence of specific early symptoms and primary resistance to chemotherapy highlighting an urgent need to improve our understanding of PDAC tumour biology. One of the key features of PDAC is the high incidence (95%) of activating mutations in the proto-oncogene KRAS. Oncogenic KRAS is known to promote resistance against intrinsic and extrinsic apoptosis, two regulated types of cell death executed by enzymatic cascades initiated by caspase activation. Counterintuitively, we find that caspase 8 - an essential enzyme in the extrinsic apoptosis cascade - is upregulated in PDAC hinting at an unknown tumour-protective function. In fact, conditional deletion of caspase 8 within a genetically-engineered mouse model of KRASG12D-driven pancreatic intraepithelial neoplasia (PanINs) ameliorated disease progression supporting this hypothesis. As caspase 8 has been shown to protect cells from aberrant necroptotic cell death, here we propose to undertake work testing the hypothesis
i) that caspase 8 deletion in pancreatic cancer triggers necroptosis
ii) how oncogenic KRAS signalling may influence necroptotic signalling and
iii) whether necroptosis induction in PDAC interacts with immunotherapy.
We thereby anticipate to identify cell death vulnerabilities in conjunction with potential markers during early PDAC progression for the development of novel treatment strategies.
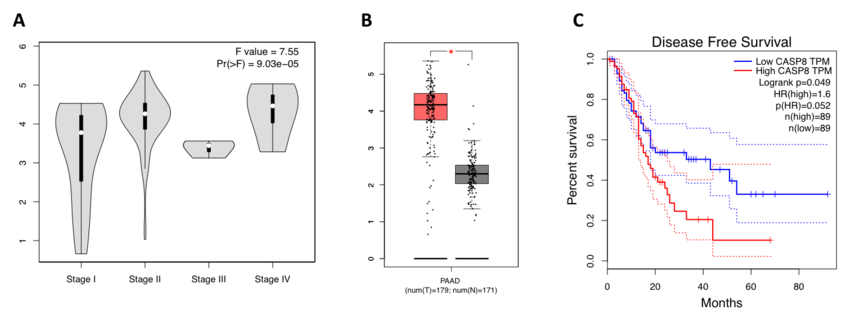
PDAC patient outlook has not improved since the 1970ies. Thereby, PDAC is amongst the few cancers with an almost identical rate of incidence and mortality. This marks PDAC as one of the cancers with unmet needs warranting the urgent requirement for novel therapeutic strategies. Here, we propose to investigate PDAC cell death biology in in-vivo model systems in order to address this need. Therapeutic approaches identified will take clinically advanced compound combinations into consideration.

Department of Translational Genomics - CECAD Research Center
CMMC - PI - A 07
show more…+49 221 478 84340
Department of Translational Genomics - CECAD Research Center
Joseph-Stelzmann-Str. 26
50931 Cologne
https://www.translational-genomics.de/research-groups/von-karstedt-lab
PostDocs:
Dr. Ariadne Androulidaki
Dr. Christina Bebber
Dr. Eric Seidel
PhD students:
Fanyu Liu
Sofya Tishina
Fatma Isil Yapici
Master students:
Büsra Aldag
Emmanuel Sarfo Gyamfi
Lejla Mulalic
Medical scientist:
Moritz Reese
Technicians:
Alina Dahlhaus
Jenny Stroh