How T lymphocytes establish long-term immune memory (Figure1) has been attributed to multiple mechanisms involving prolonged cellular longevity, post-translational regulation of key proteins, and epigenetic reprogramming of the cellular transcriptome.
In recent years, the emerging field of immunometabolism has started to unveil the role of metabolism in shaping immune function, and to reveal how modulating cell or organismal metabolism can affect immune cell differentiation. In my group at CECAD Research Center we aim at investigating how mitochondrial function is regulated in T cells in health and disease and how its dysregulation during ageing or pathology results in impaired or altered immune response. Our long-term goal is to clarify the transcriptional and microenvironmental mechanisms involved in the development of long-term immune memory and define how metabolic plasticity modulates cellular responses to stress and immune challenges.
The research in my group focuses on the role of mitochondrial metabolism, and in particular of cardiolipin (the distinctive inner mitochondrial membrane phospholipid), in shaping immunity, inflammation, and ageing. The importance of cardiolipin in mitochondrial function is evidenced not just by the X-linked inherited pathology Barth syndrome, a result of cardiolipin remodeling deficiency but also by its role in many conditions ranging from neurodegeneration to traumatic brain injury, from multiple sclerosis to heart failure. Yet we understand little about how CL alterations cause, participate or exacerbate these conditions beyond regulating OXPHOS efficiency.
We have showed how the dynamic synthesis and remodeling of cardiolipin is responsible for the metabolic and functional plasticity of CD8+ T cells during an immune response (Figure 2-3) (Corrado et al., 2020).
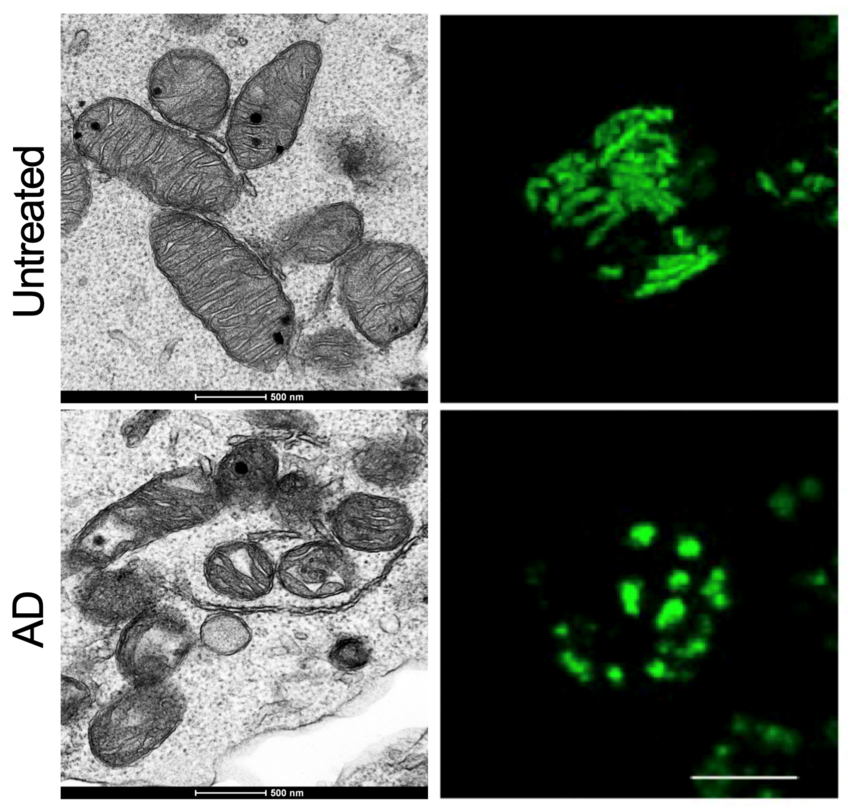
Our research also highlights CD8+ lymphopenia and functional impairments as previously uncharacterized features of Barth syndrome, where patients have Tafazzin mutations and so defective cardiolipin remodeling. Moreover, our recent work on the metabolic regulation of thymic development has shown how metabolism influences the immune system starting from the developmental stage (Corrado et al., 2021). This is important because one of the first manifestations of ageing is thymic involution. Our work shows how mitochondrial respiration impairment has long-lasting consequences on immune function and immune memory development.
In our lab, we aim at better understanding how metabolism impacts immune function in ageing, cancer or infections. The acquired knowledge about basic metabolic and signaling circuits in T cells in these conditions will lead us to harness those same pathways to improve disease prevention, treatment and outcome. Thus, our approach on immunometabolism reveals an extraordinary translational potential. In particular, our research plan has clear clinical relevance in the following three directions:
Overall, these observations show the functional nuances of metabolism in T cell immunity suggesting that modulation of cellular or organismal metabolism influence directly immune function. They also imply a possible partially immunocompromised condition in patients affected by mitochondrial diseases (both in terms of immune response to pathogens and vaccines) and open important questions:
Starting from these questions, our research program to further delve into T cell biology and physiology when mitochondrial metabolism is altered.
Sanin DE, Matsushita M, Klein Geltink RI, Grzes KM, van Teijlingen Bakke Nr, Corrado M, Kabat AM, Buck MD, Qiu J, Lawless SJ, Cameron AM, Villa M, Baixauli F, Patterson AE, Hässler F, Curtis JD, O’Neill CM, O’Sullivan D, Wu D, Mittler G, Ching-Cheng Huang S, Pearce EL, Pearce EJ: Mitochondrial membrane potential regulates nuclear gene expression in macrophages exposed to prostaglandin E2. Immunity (2018); 49 (60): 1021-1033. e6
Simula L, Pacella I, Colamatteo A, Procaccini C, Cancila V, BordI M, Tregnago C, Corrado M, Pigazzi M, Barnaba V, Tripodo C, Matarese G, Piconese S, Campello S: Drp1 controls effective T cell immune-surveillance by regulating T cell migration, proliferation, and cMyc-dependent metabolic reprogramming. Cell reports (2018); 25 (11): 3059-3073. e10
Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, Firat E, Zhu X, Nie-dermann G, Caputa G, Kelly B, Warthorst U, Rensing-Ehl A, Kyle RL, Vandersarren L, Curtis JD, Pat-terson AE, Lawless S, Grzes K, Qiu J, Sanin DE, Kretz O, Huber TB, Janssens S, Lambrecht BN, Ram-bold AS, Pearce EJ, Pearce EL: Mitochondrial priming by CD28. Cell (2017); 171 (2): 385-397 .e11
Corrado M, Mariotti FR, Trapani L, Taraborrelli L, Nazio F, Cianfanelli V, Soriano ME, Schrepfer E, Cecconi F, Scorrano L, Campello S: Macroautophagy inhibition maintains fragmented mitochondria to foster T cell receptor-dependent apoptosis. EMBO Journal (2016); 35 (16): 1793-1809.
Corrado M, Campello S: Autophagy inhibition and mitochondrial remodeling join forces to amplify apoptosis in activation-induced cell death. Autophagy (2016); 12 (12), 2496-2497
Corrado M, Scorrano L, Campello S: Changing perspective on oncometabolites: from metabolic signature of cancer to tumorigenic and immunosuppressive agents. Oncotarget (2016); 7 (29): 46692

CECAD Research Center
CMMC - PI - B 03
CMMC - PI - CAP 21
CMMC - Co-PI - B 02
+49 221 478 84167
CECAD Research Center
Joseph-Stelzmann-Str. 26
50931 Cologne
Regina Annamaria, PhD student
Sara Gjurgji, PhD student
Irma Alibashikj, Master student
Elena Potenza, Erasmus+ student