The main focus of my lab is on dissecting the molecular basis of the structure-to-function relationship of mammalian genomes. We wish to understand how chromatin integrates different signaling stimuli to control transitions between homeostatic and deregulated gene expression programs via looping regulation – particularly how changes along the linear chromatin fibre translate into spatial networks. Deciphering the general rules that govern transcriptional homeostasis will allow us to predict how a cell might respond in disease, development, or ageing.
It is now well understood that mammalian genomes are much more than mere libraries where genetic information is stored. Due to paradigm-shifting technological advances over the last decade, we now also understand that genomes are four-dimensional entities, and should be studied in 3D space and over time in order to dissect their sequence-to-structure-to-function relationship. Our work aims at furthering our understanding of 3D genomic architecture and how this is affected by and aligned to transcriptional organization in nuclei.
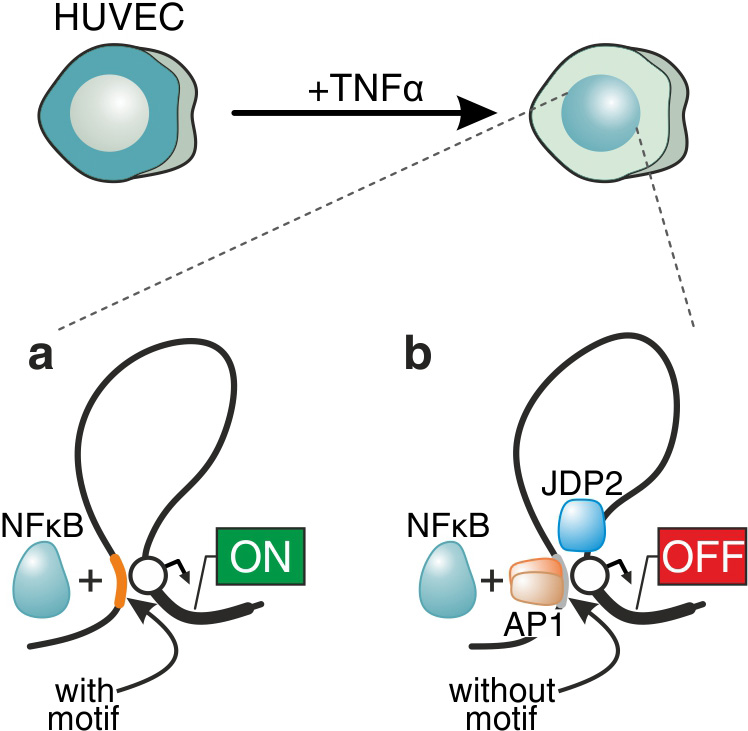
We perform comparative studies of inflammatory (TNF) versus migratory (TGF) signaling in primary endothelial cells, in order to understand how chromatin architecture allows for their diverse signaling dynamics (the former cascade unfolds within minutes, while the latter within hours). We focus on nucleosome and transcription factor occupancy landscapes, and on differences in transcriptional dynamics between the two cascades. We already described a global “priming” mechanism, whereby thousands of regions in the genome are rendered accessible following TNF stimulation, despite the absence of bound NF-κB (the factor driving the cascade); this mechanism also facilitates forthcoming changes in 3D chromatin conformation. We are currently in the process of finding out if “priming” is a universal means for preparing the genome for ensuing transcriptional programs. More recently, we discovered how non-canonical binding of transcription factors may alter their regulatory character – and turn an activator into a facilitator of gene repression (Figure 1). As a result, to further understand the molecular basis of these dynamics we initiated a project where we apply single-nucleotide resolution sequencing of nascent RNA (using our novel approach; Melnik et al, 2016), and complement it with single-cell RNA-seq (in collaboration with Karsten Rippe, Heidelberg) and single-molecule RNA FISH. As a whole, this work is bound to uncover fundamental differences in the transcriptional organization of mammalian nuclei in response to signaling and offer novel targets in these medically-relevant pathways.
We have been pursuing an integrative study of cellular senescence, the intrinsic mechanism that is responsible for the prevention of tumorigenesis, but also implicated in ageing. Here, we use different primary cell types (endothelial, lung fibroblast, and mesenchymal stem cells) in an effort to identify the common molecular backbone that signals entry into senescence. The molecular events, in 3D space and over time, driving the establishment of the senescent gene expression program remain largely unexplored, but are known to involve the activation of a proinflammatory transcriptional program. To this end, we drive the three cell types into replicative senescence, perform nascent RNA-seq, and seek to identify a common regulatory backbone between all three cell type-specific transcriptional programs, with an emphasis on differentially-regulated factors that associate with chromatin. We find genes coding for regulators of chromatin conformation being suppressed, and the levels of many transcriptional repressors being induced. Next, we use the whole-genome variant of the chromosome conformation capture technology, Hi-C (see Figure 2), to identify global changes in 3D chromatin organization. We have generated and analyzed >5 billion reads from proliferating and senescent cells, leading to interaction maps of <10-kbp resolution. Using these we identified marked reorganization at the multi-Mbp domain scale, a feature underpinned by the ubiquitous increase of the nuclear volume in senescent cells. The gain and loss of spatial interactions and the shift in topological domain boundaries correlates with non-histone chromatin constituents extruded from the nucleus into extracellular space, thus contributing to the senescence-associated secretory phenotype (SASP). Our work is the first to identify a link between the SASP and the reorganization of nuclear architecture, and it points to a particular nuclear checkpoint that prepares the cell’s response in the face of senescent arrest.
Our work on the connection between 3D chromatin folding and transcriptional organization focuses on either proinflammatory signaling, or on the onset and establishment of cellular senescence. These two processes share physiological and molecular commonalities, and their dissection will offer both a chance to identify novel effectors and potential therapeutic targets, and a deeper understanding of the 3D structure-to-function code of the human genome that still remains a challenge.

Institute of Pathology - University Medical Center Göttingen
CMMC - assoc. RG 26 (former CMMC JRG Leader)
argyris.papantonis[at]med.uni-goettingen.de
show more…+49 551 39 65734
Institute of Pathology - University Medical Center Göttingen
present adress: University Medical Center Göttingen Robert-Koch-Strasse 40
37075 Göttingen
http://zmmk-sbc.uni-koeln.de/JRG_VIII___Systems_Biology_of_Chromatin/People.html
CMMC Profile Page
Anne Zirkel (PostDoc)
Athanasia Mizi (PostDoc)
Yulia Kargapolova (PostDoc)
Lilija Brant (doctoral student)
Milos Nikolic (doctoral student)
Konstantinos Sofiadis (doctoral student)
Theodore Georgomanolis (technician)
Ruiyuan Zheng (PhD student)