SARS-CoV-2 infection leads to hyperinflammatory syndromes in a subset of patients. We show that human primary macrophages require genome-wide transcriptional modifications for pro-inflammatory signaling upon stimulation with the SARS-CoV-2 surface glycoprotein (S-protein). https://www.embopress.org/doi/full/10.15252/emmm.202114150

Dr. Alexander Simonis, assistant physician and second author of the study, Dr. Sebastian Theobald, postdoc and first author of the study and DZIF scientist Priv.-Doz. Dr. Jan Rybniker, Head of the Infectious Diseases Research Laboratory and last author of the study (from left to right). © Uniklinik Köln, Foto: Christoph Wanko
The study provides a rational for pharmacologically targeting the NLRP3 inflammasome and associated cytokines in severe cases of COVID-19. Profound alteration of macrophage gene activation and expression for several weeks to months after infection in both severe and mild COVID-19 patients can provide a better understanding of post-COVID inflammatory syndromes. Our data will help to evaluate immunogenicity of S-protein-based vaccine constructs with regard to stimulation of innate immune signaling.
SARS-CoV2 infection causes severe inflammation of the lungs and other vital organs. Why some infected individuals respond with an exaggerated immune response to the virus remains not well understood. In a new study, researchers at the Medical Faculty of University of Cologne and the University Hospital of Cologne the so-called "University Medicine Cologne" as well as the German Center for Infection Research (DZIF) are investigating the effect of the viral spike protein on the innate immune system, which is strongly associated with severity of disease. The results have now been published in the renowned scientific journal "EMBO Mol Med (2021)e14150 - https://doi.org/10.15252/emmm.202114150".
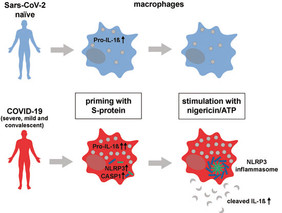
A SARS-CoV-2 infection can lead to a massive release of pro-inflammatory signalling molecules, so-called cytokines, which in some patients leads to severe organ damage and, in a chain reaction, attracts activated immune cells into the tissue. How the virus triggers the release of cytokines is not well understood. Researchers in Cologne have now been able to show that certain white blood cells (macrophages, also known as phagocytes) are massively stimulated by the viral spike protein to produce the pro-inflammatory signalling molecule interleukin 1.
However, this was only observed when macrophages from COVID-19 patients were examined in the experiments. Macrophages from people who had not yet been infected with SARS-CoV-2 did not react to the spike protein. Macrophages belong primarily to the innate immune system, which is distinguished from the acquired immune system. The latter is responsible, among other things, for antibody formation, which is also triggered by SARS-CoV-2-vaccines.
"This selective immune response of a classical signalling pathway of the innate immune system is very unusual and has not yet been described in this way,” explains the clinican scientist PD Dr. med. Dr. nat. med. Jan Rybniker, head of the Infectious Diseases Research Laboratory at the "University Medicine Cologne" and DZIF scientist. The signalling pathway of the so called inflammasome, which was investigated in this study, is also considered as a possible therapeutic target for immunomodulatory therapies in severe COVID-19 infections. A scientific basis for this approach, was identified in this work.
Interestingly, macrophages were still highly reactive towards the spike protein several weeks to months after SARS-CoV-2 infection. "Since macrophages have a very short lifespan of only a few days, this argues for changes in the genetic material of macrophage progenitor cells. We were able to detect these so-called epigenetic changes through elaborate sequencing experiments," reports Dr. Sebastian Theobald, postdoctoral researcher at the "University Medicine Cologne" and first author of the study. These profound changes found in macrophages from otherwise healthy individuals after infection with SARS-CoV-2 can now be used for a better understanding of long-term consequences of COVID-19, for example within post-COVID syndromes.
The findings are also relevant for vaccination approaches against the disease. "Our work examines the immune response towards the spike protein. Almost all currently available vaccinations are based on this protein," reports Dr. Alexander Simonis, assistant physician at the University Hospital of Cologne and second author of the study. "It is certainly beneficial for the success of the various vaccine constructs, that the spike protein leads to a strong activation of the innate immune system," Rybniker adds.
These multifaceted and in-depth investigations were only possible with the help of several collaborating partners. A total of eight research groups from the University of Cologne and the Max Planck Institute for the Biology of Aging were involved in the study, according to Dr. Jan Rybniker. The study was funded by the German Research Foundation (DFG), the German Center for Infection Research (DZIF) and the Netzwerk Universitätsmedizin (NUM).
Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19
Sebastian J Theobald, Alexander Simonis, Theodoros Georgomanolis, Christoph Kreer, Matthias Zehner, Hannah S Eisfeld, Marie-Christine Albert, Jason Chhen, Susanne Motameny, Florian Erger, Julia Fischer, Jakob J Malin, Jessica Gräb, Sandra Winter, Andromachi Pouikli, Friederike David, Boris Böll, Philipp Koehler, Kanika Vanshylla, Henning Gruell, Isabelle Suárez, Michael Hallek, Gerd Fätkenheuer, Norma Jung, Oliver A Cornely, Clara Lehmann, Peter Tessarz, Janine Altmüller, Peter Nürnberg, Hamid Kashkar, Florian Klein, Manuel Koch and Jan Rybniker
EMBO Mol Med (2021)e14150 - https://doi.org/10.15252/emmm.202114150
in bold: Scientists, who are leading as co- or principla investigators a CMMC Research Group
Scientific Contact
PD Dr. med. Dr. nat. med. Jan Rybniker
Clinic I for Internal Medicine (University Hospital of Cologne) and Center for Molecular Medicine Cologne (Medical Faculty, University of Cologne)
jan.rybniker[at]uk-koeln.de
Jan Rybniker´s Research Group Bacterial Pathogenesis and Antibiotic Drug Discovery antibiotic-drug-discovery-en/ and his CMMC CAP Project Comprehensive host-cell based antibiotic drug discovery
Source:
Press relase University Hospital Cologne - https://www.uk-koeln.de/uniklinik-koeln/aktuelles/detailansicht/lange-und-tiefgreifende-umprogrammierung-von-abwehrzellen/
Press release DZIF - German Center for Infection Reseach - https://www.dzif.de/en/long-lasting-and-profound-reprogramming-immune-cells-after-covid-19