This work contributes to filling this gap by examining downstream mediators of CR- induced resilience. CR reduces the levels of 20- hydroxyeicosatetraenoic acid, and this effect is required for the protective potential of this dietary. These findings provide new perspectives to tackle AKI in the future intervention. Kidney International . https://doi.org/10.1016/j.kint.2022.04.033
Acute kidney injury (AKI) is among the most frequent complications in hospitalized patients - especially in intensive care units - and a strong independent risk factor for chronic kidney disease and cardiovascular complications, but so far causal therapies are not available.
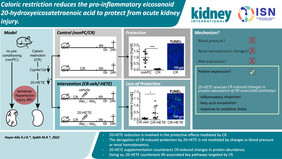
A Cologne-based research team led by Prof. Dr. Roman-Ulrich Müller has previously shown that longevity pathways can be employed by preconditioning protocols such as hypoxic preconditioning and caloric restriction (CR) to achieve a strong induction of cellular resilience in the kidney of rodents. They could show that exposing mice to a short-term caloric restriction (2-4 weeks, 40%) was highly effective in preventing AKI by ischemia-reperfusion injury (see https://doi.org/10.1016/j.trsl.2022.02.003 and https://doi.org/10.1681/asn.2019050534).
In this follow-up study the Cologne scientists discovered that CR reduces the eicosanoid 20-hydroxyeicosatetraenoic acid (20-HETE). This study - also led by Prof. Roman-Ulrich Müller – now provides important insights into the molecular mechanism underlying kidney protection by caloric restriction and offers new starting points for potential targeted interventions. The research took place at the Clinic II for Internal Medicine, the Center for Molecular Medicine Cologne (CMMC) and the Cluster of Excellence for Aging Research CECAD at the University of Cologne. The article was published in the renowned journal Kidney International. https://doi.org/10.1016/j.kint.2022.04.033
The scientists had previously examined the molecular basis of caloric restriction (CR) mediated protection using RNA-sequencing. One of the genes most strongly affected was a cytochrome required for the production of 20-HETE. Intraperitoneal supplementation of 20-HETE in preconditioned males partly abrogated the protective potential of CR depicted by creatinine values and TUNEL staining. Using targeted metabolic and proteomic analyses, in vivo imaging as well as blood pressure measurement, the Cologne scientists examined the targets of the damage-promoting effect of 20-HETE. Interestingly, their study found a partial reversal of CR-induced changes in protein – but not RNA – expression pointing towards inflammation, ER stress and lipid metabolism.
"We are very pleased that we were able to identify 20-HETE reduction as a player in the strong protective effect mediated by caloric restriction associated with renal ischemia-reperfusion injury," says K. Johanna R. Hoyer-Allo, who shares the first authorship with Martin Späth of the study. "The fact that we now know what influence 20-HETE has on protein abundance in cells offers new starting points for potential targeted interventions in the future," adds Martin Späth, shared first author of the study.
The Cologne scientists show the great potential of dietary interventions to prevent kidney disease. "The huge potential of dietary interventions to prevent kidney disease are often much greater than the effects of drugs. Our study fills an important gap that will further facilitate to find the mechanisms behind this phenomenon and pave the way towards clinical application,” says Prof. Dr. Roman Müller.
Publication
Title: Caloric restriction reduces the pro-inflammatory eicosanoid 20-hydroxyeicosatetraenoic acid to protect from acute kidney injury
Authors: Karla Johanna Ruth Hoyer-Allo125, Martin Richard Späth125, Susanne Brodesser2, Yiyi Zhu3, Julia Binz-Lotter1,2, Martin Höhne1,2, Hella Brönneke3, Katrin Bohl1,2, Marc Johnse1,2, Torsten Kuback1,2, Katharina Kiefer1,2, Lisa Seufert1,2, Felix Carlo Koehler1,2, Franziska Grundmann1, Matthias J. Hackl1,2, Bernhard Schermer1,2, Jens Brüning2,3,4, Thomas Benzing1,2, Volker Burst1, Roman-Ulrich Müller1,2
Affilliations: 1Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany / 2Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany / 3Max-Planck-Institute for Metabolism Research, University of Cologne, Faculty of Medicine and University Hospital Cologne Cologne, Germany / 4Policlinic for Endocrinology, Diabetes and Preventive Medicine (PEPD), University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany
Journal: Kidney Internationalhttps://doi.org/10.1016/j.kint.2022.04.033
Contact:
Prof. Dr. Roman-Ulrich Müller
Clinic II of Internal Medicine, Center for Molecular Medicine Cologne (CMMC) and CECAD Cluster of Excellence in Aging Research
Faculty of Medicine, University of Cologne
roman-ulrich.mueller[at]uk-koeln.de