Tissue stem cells (SC) show a multitude of mechanisms to avoid or repair damage, including low mitochondrial mass and minimal use of oxidative metabolism. However, mitochondrial mass must be dramatically expanded during SC differentiation, thus, intactness of mtDNA is essential. The aim of this project is to test (i) if mtDNA is only rarely replicated in quiescent muscle SCs or, alternatively, (ii) that it is purified during recruitment, i.e. wt mtDNA copies are kept in MuSCs while ΔmtDNA is passed to the progeny.
Skeletal muscle contains a quiescent stem cell pool (satellite cells; MuSCs) which is only recruited after injury, but also after strenous, albeit physiological exercise. The regenerative capacity of MuScs declines with age and recent studies have suggested that progressive aging-related and cell-intrinsic alterations play a greater role in this functional decline than previously thought. Those cell-intrinsic alterations are discussed as important factors contributing to aging-induced sarcopenia, defined as a loss of muscle mass and function. Understanding the processes causing those aging-related alterations in MuSCs is thus essential to establish new strategies to delay the development of sarcopenia, e.g. by eccentric exercise regimes, which have been reported to be beneficial in humans.
SCs in general have a multitude of mechanisms to avoid or repair damage to all cellular structures. For example, hematopoietic SCs have only few mitochondria and few copies of mitochondrial DNA (mtDNA), and are highly glycolytic, a metabolic strategy thought to minimize collateral damage by oxidative metabolism and maintain stemness. Nevertheless, both mitochondrial mass and mtDNA copy number must be expanded during differentiation of activated MuSc into mature fibers, which are largely dependent on mitochondrial metabolism. Thus, intactness of the mtDNA in MuSC is essential for normal skeletal muscle regeneration, but little is known about the mechanisms maintaining its integrity, which is likely to be compromised during aging.
In order to study the cell specific consequences of mtDNA deletions, we have generated a mouse strain which expresses a dominant-negative mutant of the mitochondrial helicase Twinkle (Rosa26-Stop-construct, point mutation K320E), strongly enhancing the generation of ΔmtDNA species, as observed in tissues of affected patients.
Expression of this construct in the myocardium (Ckmm-Cre) leads, as intended, to a tissue mosaic with only few COXneg cells, which however causes severe arrhythmia, again a typical and important age-related disorder (Baris, …, Wiesner 2015). Expression of K320E-Twinkle in the epidermis (K14-Cre) causes severe inflammation with upregulation of a variety of pro-inflammatory cytokines (Weiland,…, Wiesner & Baris, 2018). Both studies therefore unequivocally showed that mitochondrial tissue mosaics are indeed of pathophysiological relevance.
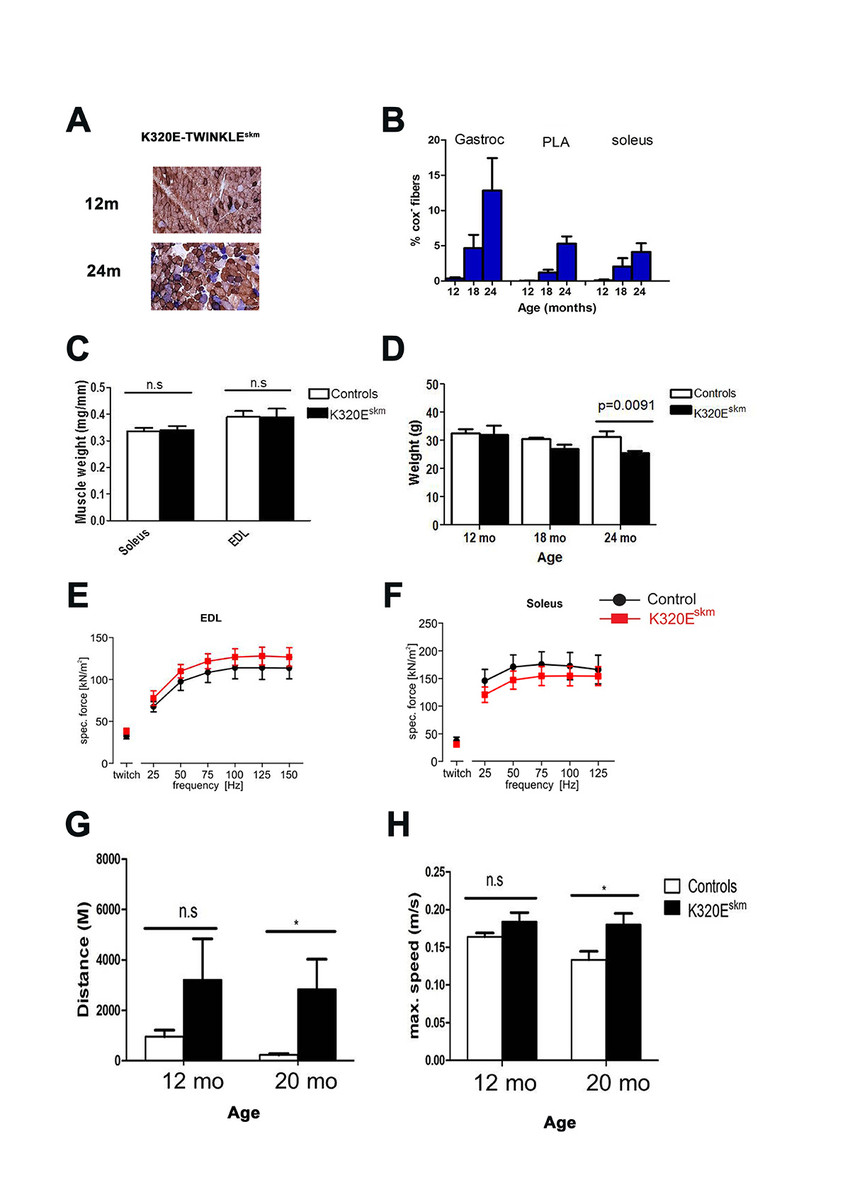
We have expressed K320E-Twinkle in differen-tiated muscle cells driven by Mlc1f-Cre. Those mice (K320E-Twinkleskm) display a remarkable accumulation of COXneg muscle fiber segments (Fig. 1 A,B), but surprisingly do not show hallmark features of sarcopenia (loss of muscle mass and function) as expected (Fig. 1C- H). Transcriptomic and proteomic studies are underway to determine potential compensatory mechanisms in these mice.
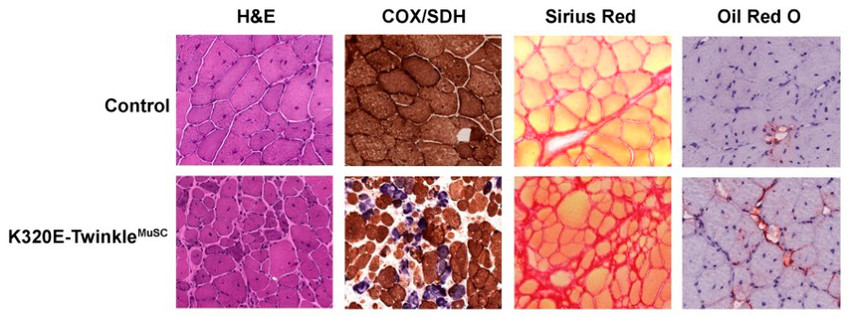
Next, we have induced ΔmtDNA in muscle satellite cells (MuSCs) and study (i) possible mechanisms maintaining mtDNA integrity and (ii) whether these deteriorate during aging. For this purpose, we express K320E-Twinkle specifically in MuScs driven by Pax7-CreERT. When the tibialis anterior (TA) muscle of those mice (K320E-TwinkleMuSC) is damaged following cardiotoxin (CTX) injection, its regeneration from MuScs is dramatically impaired when compared to muscle regenerated in control mice (Fig. 2). This shows that mitochondrial dysfunction induced by mtDNA deletions is an important intrinsic factor impairing the regenerative capacity of MuSC.
The decline in regenerative capacity of MuSCs with age contributes to the development of sarcopenia. Sarcopenia leads to physical disability, but more importantly to an increased incidence of falls followed by hospitalization, which may result in premature death. Loss of muscle mass may also contribute to insulin resistance typical for old age. Understanding aging-related alterations in mechanisms that maintain mtDNA integrity in MuSCs is essential to establish new strategies to delay the development of sarcopenia.
Weiland, D., Brachvogel, B., Hornig-Do, H.-T., Neuhaus, J.F.G., Holzer, T., Tobin, D.J., Niessen, C.M., Wiesner, R.J.# and Baris, O.R. (2018). Imbalance of mitochondrial respiratory chain complexes in the epidermis induces severe skin inflammation. J Invest Dermatol 138, 132-140. #corresponding author
Kloepper, J. E., O.R. Baris, K. Reuter, K. Kobayashi, D. Weiland, S. Vidali, D. J. Tobin, C. Niemann, R.J. Wiesner# and R. Paus (2015). Mitochondrial function in murine skin epithelium is crucial for hair follicle morphogenesis and epithelial-mesenchymal interactions. J Invest Dermatol 135, 679-89. #corresponding author
Baris OR, Ederer S, Neuhaus JFG, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stöckigt F, Schrickel JW, and Wiesner RJ (2015). Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metab. 21, 667-677.
Franko A, von Kleist-Retzow JC, Neschen S, Wu M, Schommers P, Böse M, Kunze A, Hartmann U, Sanchez-Lasheras C, Stöhr O, Huntgeburth M, Brodesser S, Irmler M, Beckers J, de Angelis MH, Paulsson M, Schubert M, and Wiesner RJ (2014). Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J. Hepatol. 60. 816-823.
Neuhaus JFG, Baris OR, Hess S, Moser N, Schröder H, Chinta SJ, Andersen JK, Kloppenburg P, and Wiesner RJ (2014). Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain 137. 354-365.
Holzer, T., Probst, K., Etich, J., Auler, M., Georgieva, V.S., Bluhm, B., Frie, C., Heilig, J., Niehoff, A., Nuchel, J., Plomann, M., Seeger, J.M., Kashkar, H., Baris, O.R., Wiesner, R.J., and Brachvogel, B. (2019). Respiratory chain inactivation links cartilage-mediated growth retardation to mitochondrial diseases. J Cell Biol 218, 1853-70.
Pla-Martin, D., and Wiesner, R.J. (2019). Reshaping membranes to build mitochondrial DNA. PLoS Genet 15, e1008140.
Weiland D, Brachvogel B, Hornig-Do HT, Neuhaus JFG, Holzer T, Tobin DJ, Niessen CM, Wiesner RJ, and Baris OR (2018). Imbalance of Mitochondrial Respiratory Chain Complexes in the Epidermis Induces Severe Skin Inflammation. J Invest Dermatol 138, 132-140.
Franko A, Kunze A, Bose M, von Kleist-Retzow JC, Paulsson M, Hartmann U, and Wiesner RJ (2017). Impaired Insulin Signaling is Associated with Hepatic Mitochondrial Dysfunction in IR+/--IRS-1+/- Double Heterozygous (IR-IRS1dh) Mice. Int J Mol Sci 18.
Neuhaus JF, Baris OR, Kittelmann A, Becker K, Rothschild MA, and Wiesner RJ (2017). Catecholamine Metabolism Induces Mitochondrial DNA Deletions and Leads to Severe Adrenal Degeneration during Aging. Neuroendocrinology 104, 72-84.
Information from this funding period will not be updated anymore. New research related information is available here.
Maintenance of mitochondrial DNA integrity in epidermal keratinocytes during aging caused by internal and external insults

Institute of Vegetative Physiology
CMMC - PI - C 17
rudolf.wiesner[at]uni-koeln.de
show more…+49 221 478 3610
+49 221 478 3538
Institute of Vegetative Physiology
Robert-Koch-Str. 39
50931 Cologne
http://physiologie.uni-koeln.de/21674.html
CMMC Profile Page
Julia Boix-Tarin, PhD (PostDoc)
David Pla-Martin, PhD (PostDoc)
Sammy Kimoloi (doctoral student)
Thomas Pass (doctoral student)
Maria Bust (technician)
Katrin Lanz (technician)
Nadine Niehoff (technician)