Astrocytes have emerged for playing key roles in tissue remodeling during brain repair, however the underlying mechanisms remain poorly understood. We show that acute injury and blood-brain barrier (BBB) disruption trigger the formation of a prominent mitochondrial-enriched compartment in astrocytic end-feet which regulates local metabolic signalling domains. Disruption of this mitochondrial clustering impaired vascular recovery, indicating an important role of astrocytes in tissue repair.
By virtue of their intimate specialized connection with neurons and the brain vasculature, astrocytes regulate essential aspects of brain energy metabolism but are also invariably involved in most neurodegenerative and inflammatory disorders.In particular,astrocytes have emerged for playing key roles in tissue remodeling during brain repair, and recent workindicates that they have the capability to strongly influence the extent of axon regeneration following injury. However, to which extent astrocytes can modulate the recovery of the vascular compartment is unclear.
In this project we addressed the key question whether astrocytes may play an active role in the local structural remodeling of the microvasculature in a model of stab-wound injury. Using a combination of state-of-the-art imaging, genetic and proteomic approaches we investigated with unprecedented sub-cellular resolution the changes in organelle networks experienced by reactive astrocytes in an injury setting in vivo, whether these may have any role in regulating tissue repair and if so, what are the underlying mechanisms. We found that the remodelling of the mitochondrial network in astrocytes reacting to injury and BBB disruption is part of a metabolic adaptive response that culminates in the generation of a spatially-defined mitochondrial-enriched domain in perivascular end-feet, and which is ultimately required for vascular repair. In particular, we identified this metabolic program to be regulated by mitochondrial fusion and mitochondrial tethering to ER membranes, the latter being particularly abundant in astrocytic end-feet (Fig. 1A-D).
Astrocyte-specific deletion of the outer mitochondrial membrane GTPase mitofusin 2 (Mfn2), which regulates mitochondrial fusion dynamics, prevented injury-induced perivascular accumulation of mitochondria, altered the extent of mitochondria-ER tethering and led to disrupted mitochondrial Ca2+ uptake in astrocyte end-feet, ultimately impairing the recovery of the vascular network in the injured area (Fig. 1E-H). Importantly, by delivering astrocyte-specific adeno-associated viruses (AAVs) encoding for synthetic linkers that specifically anchor mitochondria to ER membranes, thereby enhancing perivascular accumulation of astrocytic mitochondria, we could demonstrate that vascular repair can be restored in absence of mitochondrial fusion. These results establish a new mechanism for astrocytic mitochondrial fusion in orchestrating the metabolic adaptations of brain tissue in vivo and unravel a key role for astrocytes in sustaining microvasculature remodelling during repair.
While this study reveals the essential role of a “metabolic” reactive state in astrocytes in regulating the functional recovery of damaged brain vasculature, with obvious key implications for a number of neurological diseases characterized by vascular conditions, it also raises a number of important questions. In particular, as complimen-tary experiments indicate that the effects observed here do not primarily depend upon mitochondrial oxidative phosphorylation (OXPHOS), the specific mechanisms by which a local accumulation of astrocytic mitochondria around vessels may facilitate vascular repair remain to be identified. For example, is there a local gradient of signalling molecules or metabolites which fuel the formation of new vessels, and what exactly this signalling may be that depends from a local bulk accumulation of mitochondria? How do changes in the reactive state of perivascular astrocytes translate into metabolic changes at the level of the whole vascular unit? Future work will address these specific questions.
1. Göbel J., et al.(2019).Mitochondrial fusion in reactive astrocytes coordinates local metabolic domains to promote vascular repair. BioRxiv; doi: doi.org/10.1101/657999.
2. Göbel, J., et al.(2018). Spatiotemporal control of mitochondrial network dynamics in astroglial cells. Biochem Biophys Res Commun 500, 17-25.
3. Motori, E., et al.(2013). Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab 18, 844-859.
Riedemann, S., Sutor, B., Bergami, M., and Riedemann, T. (2019). Gad1-promotor-driven GFP expression in non-GABAergic neurons of the nucleus endopiriformis in a transgenic mouse line. J Comp Neurol 527, 2215-32.
Sprenger, H.G., Wani, G., Hesseling, A., Konig, T., Patron, M., MacVicar, T., Ahola, S., Wai, T., Barth, E., Rugarli, E.I., Bergami, M., and Langer, T. (2019). Loss of the mitochondrial i-AAA protease YME1L leads to ocular dysfunction and spinal axonopathy. EMBO Mol Med 11.
Gobel J, Motori E, and Bergami M (2018). Spatiotemporal control of mitochondrial network dynamics in astroglial cells. Biochem Biophys Res Commun 500, 17-25.
Jahn HM, and Bergami M (2018). Critical periods regulating the circuit integration of adult-born hippocampal neurons. Cell Tissue Res 371, 23-32.
Sprenger HG, Wani G, Hesseling A, Konig T, Patron M, MacVicar T, Ahola S, Wai T, Barth E, Rugarli EI, Bergami M, and Langer T (2018). Loss of the mitochondrial i-AAA protease YME1L leads to ocular dysfunction and spinal axonopathy. EMBO Mol Med 10.15252/emmm.201809288.
Engelhardt E, Gobel J, Jahn HM, and Bergami M (2017). Dissecting the role of NEMO/NF-kappa B in astrocytes reacting to injury and inflammation. Glia 65, E450-E.
Gobel J, Ghanem A, Motori E, Jahn HM, Wani G, Conzelmann KK, and Bergami M (2017a). Perivascular remodeling of astrocytic ER and mitochondrial networks following brain injury. Glia 65, E526-E.
Gobel J, Motori E, and Bergami M (2017b). Spatiotemporal control of mitochondrial network dynamics in astroglial cells. Biochem Biophys Res Commun 10.1016/j.bbrc.2017.06.191.
Jahn HM, and Bergami M (2017). Critical periods regulating the circuit integration of adult-born hippocampal neurons. Cell Tissue Res 10.1007/s00441-017-2677-x.
Information from this funding period will not be updated anymore. New research related information is available here.
Role of metabolic signaling at the glio-vascular interface during brain injury and repair

CECAD Cologne
CMMC - PI - A 02
show more…+49 221 478 84250
+49 221 478 78910
CECAD Cologne
Joseph-Stelzmann-Str. 26
50931 Cologne
Jana Göbel (doctoral student)
Esther Engelhardt (doctoral student)
Vignesh Sakthivelu (PostDoc)
Hannah Jahn (PostDoc)
Gulzar Wani (doctoral student)
Sandra Wendler (doctoral student)
Kristiano Ndoci (doctoral student)
Milica Jevtic (lab manager)
Timucin Öztürk (technician)
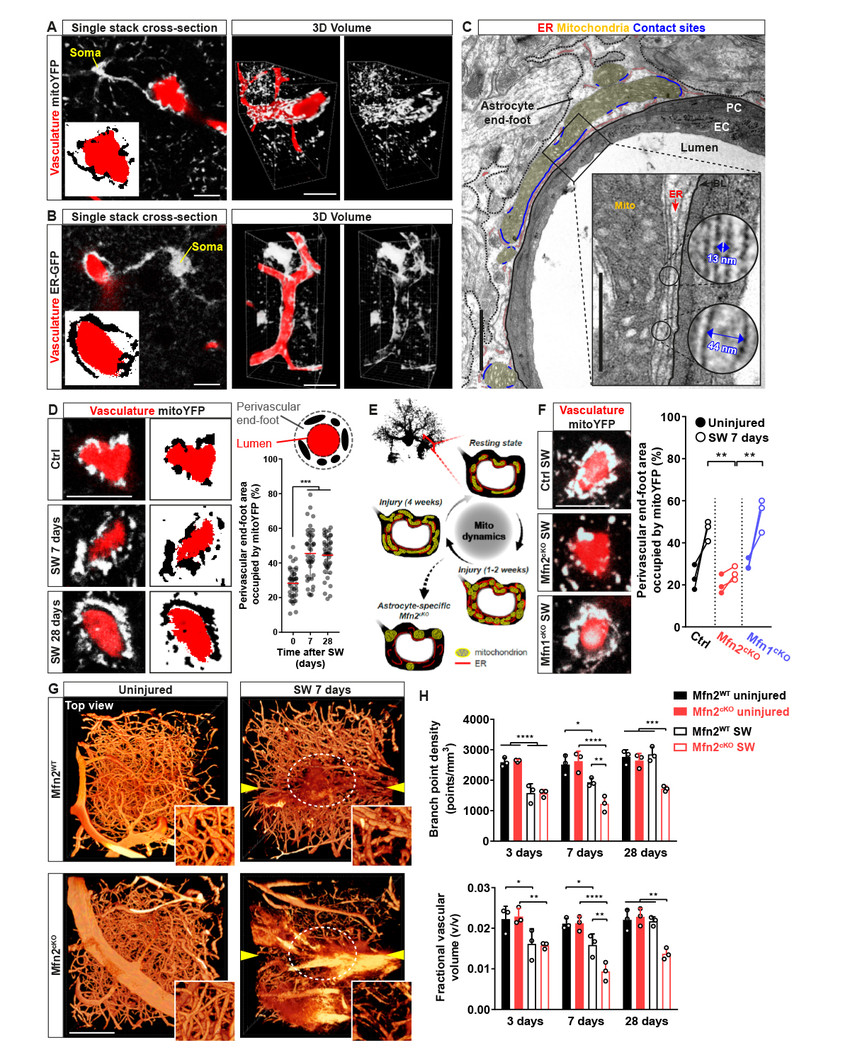
Figure 1.Localmitochondrial enrichment in astrocytic perivascular end-feet depends on mitochondrial fusion dynamics and is required for vascular repair.(A-B) Examples of astrocytes expressing ER-GFP or mitoYFP wrapping around dextran-labelled vessels. Side panels show a 3D rendering of the same astrocytes. Bars, 10 and 20 µm. (C) EM picture of a vessel cross-section showing the astrocytic end-foot. The inset shows mitochondria-ER contact sites lining the basal lamina. Bars, 2 and 1 µm. (D) Examples and quantification of perivascular astrocytic mitoYFP accumulation at 7 and 28 days after stab-wound injury (SW). Bar, 10 µm. (E) Model showing the mitochondrial remodeling in astrocytic end-feet and the expected phenotype following Mfn2 deletion. (F) Examples and quantification perivascular astrocytic mitoYFP accumulation following injury in Mfn2cKO and, for comparison, Mfn1cKO animals. Bar, 10 µm. (G) Top views of the vascular network (dextran-labelled) in reconstructed portions of control and Mfn2cKO brain cortices. Arrowheads point to the lesion tracks. Insets depict zooms of the lesioned core region (circled in white). Bar, 200 µm. (H) Time course quantification of vascular density after injury in control and Mfn2cKO mice. *, p < 0.05, **, p < 0.01, ***, p < 0.001. Adapted from Göbel et al. 2019 BioRxiv.