The aim of the Institute for Molecular and Behavioral Neuroscience is to study molecular mechanisms underlying disease-associated changes in cellular excitability using transgenic mouse models. We studied the function of HCN channels in early forebrain development by creating a mouse line with ablated h-current, and by examining the effect of HCN inhibitor on neural stem cells. Our results indicate that HCN channel function in early embryonic period is essential for normal cortical development.
Developmental and evolutionary expansion of the cerebral cortex relies on the controlled division of neural stem and progenitor cells, to which the membrane potential is suggested to contribute. HCN channels, which mediate I(h), a voltage-dependent non-selective cation current, depolarize the membrane potential towards the action potential threshold and, thus, importantly contribute to the dynamic control of the resting membrane potential in neurons.
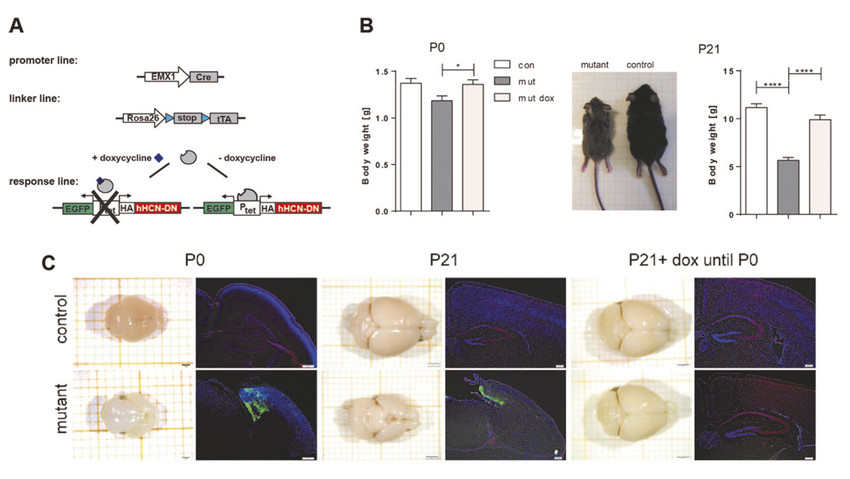
We recognized the previously unknown role of HCN channels in regulating the proliferation of neural progenitors during forebrain development. We demonstrate expression of HCN subtypes, particularly HCN3 and HCN4, in human, mouse, and rat neural progenitors of the cerebral cortex. Mice with early embryonic ablation of HCN function restricted to the forebrain under the control of the EMX1 promoter had a pronounced microcephaly phenotype associated with a decrease in proliferation and premature expression of glial markers, which was absent when HCN-channel function was ablated in newborn post-mitotic cortical neurons using the Nex promoter. Pharmacological blockade of I(h) in neural stem cell cultures from rat cortices (NSC) by ZD7288 revealed a dose-dependent proliferation impairment. In addition, single-cell transcriptome analysis of ZD7288-treated and untreated rat NSCs revealed an accumulation of treated cells in the G1 phase of the cell cycle. These findings suggest that impaired I(h)-mediated membrane potential dynamics resulted in G1-phase lengthening, which is associated with decreased proliferation and premature differentiation of neuronal progenitors.
Our current data indicate that proper HCN channel function is important to forebrain development, and that mutations in HCN-channel genes could lead to brain malformations. We now plan to study the databases with patients that have brain malformations coupled with HCN subunit gene mutations. We intend to show, after we provided proof-of-principle that HCN channel function is essential for proliferation of neural progenitors, that our data are clinically relevant. In another line of study we use calcium imaging and voltage-sensitive dyes to demonstrate mechanistic coupling between the h-current inhibition and cellular proliferation. We also have data indicating that HCN inhibition plays a role in microglia proliferation and determination of microglial phenotype. Finally, we conduct the experiments to show the effects of M-current blockade on neural stem cells and forebrain morphology.
Schlusche, A.K., Vay, S.U., Sandke, S., Campos-Martin, R., Kleinenkuhnen, N., Florio, M., Huttner, W., Tresch, A., Roeper, J., Stockebrand, M., Rueger, M.A., Jakovcevski, I., Isbrandt, D. (2018). Embryonic loss of HCN/h-channel function in mouse forebrain results in impaired neural progenitor proliferation and microcephaly. FENS abstract F18-3491.
Schmitz L, Kuglin R, Bae-Gartz I, Janoschek R, Appel S, Mesaros A, Jakovcevski I, Vohlen C, Handwerk M, Ensenauer R, Dotsch J, and Hucklenbruch-Rother E (2018). Hippocampal insulin resistance links maternal obesity with impaired neuronal plasticity in adult offspring. Psychoneuroendocrinology 89, 46-52.
Ortega JA, Memi F, Radonjic N, Filipovic R, Bagasrawala I, Zecevic N, and Jakovcevski I (2018). The Subventricular Zone: A Key Player in Human Neocortical Development. Neuroscientist 24, 156-170.
Vulovic M, Divac N, and Jakovcevski I (2018). Confocal Synaptology: Synaptic Rearrangements in Neurodegenerative Disorders and upon Nervous System Injury. Front Neuroanat 12, 11.
Wunderlich G, Abicht A, Brunn A, Daimaguler HS, Schroeter M, Fink GR, Lehmann HC, and Cirak S (2018). [Congenital myasthenic syndromes in adulthood: Challenging, rare but treatable]. Nervenarzt10.1007/s00115-018-0562-9.
Information from this funding period will not be updated anymore. New research related information is available here.
Developmental-stage specific analysis of molecular disease mechanisms in genetic epileptic encephalopathies

Institute for Molecular and Behavioral Neuroscience
CMMC - PI - assoc. 39
show more…+49 221 478 32732
+49 221 478 32400
Institute for Molecular and Behavioral Neuroscience
Kerpener Str. 62
50937 Cologne

Dept. of Neurology / RG location - LFI Building
Co-Principal Investigator C 8
michael.schroeter[at]uk-koeln.de
show more…+49 221 478 87239
+49 221 478 32400
Dept. of Neurology / RG location - LFI Building
Kerpener Str. 62
50924 Cologne
Anna K. Schlusche (PostDoc)
Malte Stockebrand (PostDoc)
Sabine U. Vay (PostDoc)