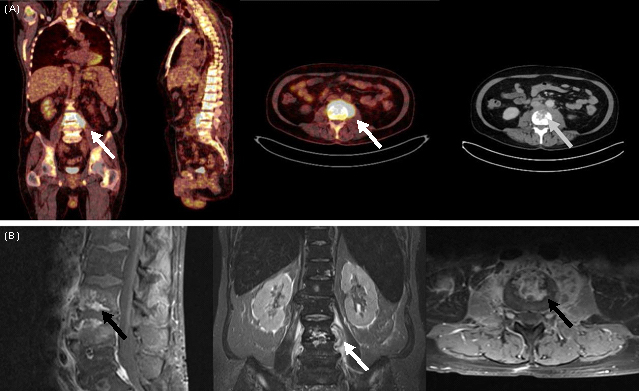
In patients, a variety of risk factors predispose for different invasive fungal infections. Common invasive fungal infections are invasive candidiasis, invasive aspergillosis, and mucormycosis. Patients with well-defined profound immunosuppression are at high risk for invasive aspergillosis and mucormycosis, whereas invasive candidiasis occurs in immunocompetent patients, too.
Current standard diagnostics for invasive fungal infections such as blood culture, bronchoalveolar lavage, tissue biopsies and serological assays are sensitive to errors, sometimes unspecific, invasive and often contraindicated. The lack of reliable diagnostics along with high mortality lead to complex treatment strategies by means of prophylaxis, fever-driven, diagnosis-driven, and targeted treatment approaches.
With the development of the antigen-reactive T cell assay a non-invasive diagnostic allows fast and valid identification of pathogens to genus and species level when standard tests remain negative or patients’ comorbidities forbid invasive diagnostics.
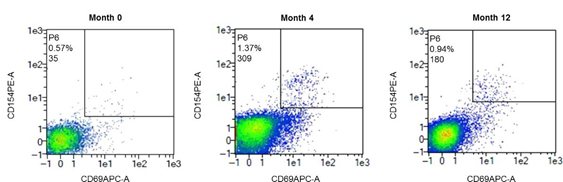
We use peripheral blood mononuclear cells (PBMCs) from patients’ full blood and challenge them with fungal lysates. If the PBMCs encounter a repetitive fungal challenge, we identify fungus-reactive CD4+ T cells in the patient’s blood via flow cytometry.
The current project is advanced to further bacterial and fungal pathogens for clinical diagnostics and to investigate the cellular immune response in cooperation with the AG Klinische Mikrobiomforschung (PD Dr. M. Vehreschild) and AG Immunologie der HIV-Infektion (PD Dr. C. Lehmann).
Invasive fungal infections are associated with high morbidity and mortality in immunosuppressed and immunocompetent patients. With the development of new diagnostic tools, we want to overcome the problem of false negative standard diagnostics to facilitate targeted treatment and thus improve patient outcome.We receive patient samples from multiple sites in Germany through our FungiResearch network (http://fungiresearch.uni-koeln.de) and act as the lab of the European Excellence Center of Medical Mycology (https://www.ecmm.info).
Bacher, P., Hohnstein, T., Beerbaum, E., Rocker, M., Blango, M.G., Kaufmann, S., Rohmel, J., Eschenhagen, P., Grehn, C., Seidel, K., Rickerts, V., Lozza, L., Stervbo, U., Nienen, M., Babel, N., Milleck, J., Assenmacher, M., Cornely, O.A., Ziegler, M., Wisplinghoff, H., Heine, G., Worm, M., Siegmund, B., Maul, J., Creutz, P., Tabeling, C., Ruwwe-Glosenkamp, C., Sander, L.E., Knosalla, C., Brunke, S., Hube, B., Kniemeyer, O., Brakhage, A.A., Schwarz, C., and Scheffold, A. (2019). Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 176, 1340-55 e15.
Salmanton-Garcia, J., Seidel, D., Koehler, P., Mellinghoff, S.C., Herbrecht, R., Klimko, N., Racil, Z., Falces-Romero, I., Ingram, P., Benitez-Penuela, M.A., Rodriguez, J.Y., Desoubeaux, G., Barac, A., Garcia-Vidal, C., Hoenigl, M., Mehta, S.R., Cheng, M.P., Klyasova, G., Heinz, W.J., Iqbal, N., Krause, R., Ostermann, H., Penack, O., Schalk, E., Sheppard, D.C., Willinger, B., Wisplinghoff, H., Vehreschild, J.J., Cornely, O.A., Vehreschild, M., and FungiScope, E.I.W.G. (2019). Matched-paired analysis of patients treated for invasive mucormycosis: standard treatment versus posaconazole new formulations (MoveOn). J Antimicrob Chemother 10.1093/jac/dkz344.
Steinbach, A., Cornely, O.A., Wisplinghoff, H., Schauss, A.C., Vehreschild, J.J., Rybniker, J., Hamprecht, A., Richter, A., Bacher, P., Scheffold, A., and Koehler, P. (2019). Mould-reactive T cells for the diagnosis of invasive mould infection-A prospective study. Mycoses 62, 562-9.
Information from this funding period will not be updated anymore. New research related information is available here.

CECAD / Clinical Trials Center Cologne
CMMC - PI - assoc. RG 28
show more…+49 221 478 6494
+49 221 478 86465
CECAD / Clinical Trials Center Cologne
Gleueler Str. 269
50935 Cologne
http://www.cecad.uni-koeln.de/translational-research/clinical-trials/