Despite decades of research, lung cancer remains the most common cause of cancer-related death. Most recently multiple promising approaches, reigniting a patient´s own adaptive anti-tumor immunity, fueled hope to identify novel treatment regimens for this aggressive tumor. Here, we will employ highly predictive in vivo models for most efficient bench-to-bedside translation of multimodal anticancer therapy consisting of CDK4/6 inhibition, radiation therapy, and immune checkpoint inhibition.
Worldwide, lung cancer accounts for over 1 million deaths per year – and, consequently, is the most common cause of cancer-related death. Although targeted therapies for lung adenocarcinomas (approx. 40% of all lung cancers) have been developed (e.g. Erlotinib, Alectinib), aggressive KRAS-driven lung adenocarcinoma remains refractory to targeted treatment strategies until today. KRAS mutations are frequently associated with concurrent loss of key tumor suppressor genes, such as TP53 or STK11/LKB1 in this tumor type. Currently, increased response rates of lung cancers are observed when treated with immune checkpoint inhibitors rearousing the patient´s own adaptive anti-tumor immunity. However, only a minority of patients responds yet, and it is highly likely that application of multimodal therapy is required to tackle KRAS-driven lung cancer. Here, we address the question whether radiation therapy (RTx) and CDK4/6 inhibitors act synergistically to sensitize Kras-driven lung adenocarcinoma towards immune checkpoint inhibitors (ICIs).
In previous work, we examined whether antineoplastic small molecules were able to enhance efficacy of existing immunotherapies in treating cancer. We found that specifically CDK6 depletion enhanced IL-2 secretion of T cells. The CDK4/6 inhibitors Palbociclib and Trilaciclib (G1T28) showed significantly more selectivity for the target protein compared to others. Treatment efficacy studies in an immune-competent genetically engineered mouse model of human non-small cell lung cancer: KrasLSL-G12D;Trp53fl/fl (KP) showed tumor reduction upon transient Trilaciclib application (Fig. 1, left panel). However, CDK4/6 inhibition alone was not sufficient to eradicate tumors. Using the murine syngeneic colon adenocarcinoma models MC38 and CT26, we evaluated the ability of CDK4/6 inhibition to complement PD-1 blockade. This dual combination nearly eliminated tumor growth (Fig. 1, middle and right panel). However, this effect did not last and tumors started to regrow quickly.
Our preclinical data of combinatorial treatment with RTx and PD-1 antibodies on a Kras-driven murine NSCLC model showed significant tumor shrinkage (up to 70%) and durable antitumor efficacy (stable with very low tumor burden after completion of 12 weeks (Fig. 2, left panel)), whereas the single treatments only induced stable disease for a shorter time period (Fig. 2, right panel). Despite these encouraging data, complete tumor eradication could not be achieved. In a recent study, Whittaker and colleagues found a significant survival advantage in an orthotopic glioblastoma mouse model when CDK4/6 inhibition (in this case Palbociclib) and RTx were combined (Whittaker et al., Cell Death Discov, 2017). We believe that transient modulation of the tumor microenvironment by CDK4/6 inhibition prior to RTx could render this treatment modality more efficient.
Despite the increasing body of evidence highlighting the clinical potential of antitumor efficacy of combined RTx and ICI treatment, less responsive lung cancer types exist suggesting for dominant immune evading mechanisms. Significant amounts of infiltrating regulatory T cells and neutrophils, which are associated with facilitating the suppression of the antitumor response through various tolerance induction and tissue homeostatic mechanisms, are found in murine and human tumors. We aim to investigate whether specific targeting of these cells holds promise for advanced cancer therapy to ultimately help clinicians to design informed patient-tailored clinical studies for rapid translation in the human setting.
Deng, J., Wang, E.S., Jenkins, R.W., Li, S., Dries, R., Yates, K., Chhabra, S., Huang, W., Liu, H., Aref, A.R., Ivanova, E., Paweletz, C.P., Bowden, M., Zhou, C.W., Herter-Sprie, G.S., Sorrentino, J.A., Bisi, J.E., Lizotte, P.H., Merlino, A.A., Quinn, M.M., Bufe, L.E., Yang, A., Zhang, Y., Zhang, H., Gao, P., Chen, T., Cavanaugh, M.E., Rode, A.J., Haines, E., Roberts, P.J., Strum, J.C., Richards, W.G., Lorch, J.H., Parangi, S., Gunda, V., Boland, G.M., Bueno, R., Palakurthi, S., Freeman, G.J., Ritz, J., Haining, W.N., Sharpless, N.E., Arthanari, H., Shapiro, G.I., Barbie, D.A., Gray, N.S., Wong, K.K. (2018) CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8(2):216-233.
Herter-Sprie, G.S.*, Koyama, S.*, Korideck, H., Hai, J., Deng, J., Li, Y.Y., Buczkowski, K.A., Grant, A.K., Ullas, S., Rhee, K., Cavanaugh, J.D., Poudel Neupane, N., Christensen, C.L., Herter, J.M., Makrigiorgos, G.M., Hodi, F.S., Freeman, G.J., Dranoff, G., Hammerman, P.S., Kimmelman, A.C., Wong, K.K. (2016) Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight.1(9):e87415. *equal contribution
Koyama, S., Akbay, E.A., Li, Y., Aref, A.R., Skoulidis, F., Herter-Sprie, G.S., Buczkowski, K.A., Liu, Y., Awad, M.M., Denning, W.L., Diao, L., Wang, J., Parra-Cuentas, E.R., Wistuba, I.I., Soucheray, M., Thai, T.C., Asahina, H., Kitajima, S., Altabef, A., Cavanaugh, J.D., Rhee, K., Gao, P., Zhang, H., Fecci, P.E., Shimamura, T., Hellmann, M.D., Heymach, J.V., Hodi, F.S., Freeman, G.J., Barbie, D.A., Dranoff, G., Hammerman, P.S., Wong, K.K. (2016). STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 76(5):999-1008.
Herter, J.M., Grabie, N., Cullere, X., Azcutia, V., Rosetti, F., Bennett, P., Herter-Sprie, G.S., Elyaman, W., Luscinskas, F.W., Lichtman, A.H., Mayadas, T.N. (2015). AKAP9 regulates activation-induced T lymphocyte retention at sites of inflammation.Nat Commun. 6:10182.
Herter-Sprie, G.S., Korideck, H., Christensen, C.L.,Herter, J.M., Rhee, K., Berbeco, R.I., Bennett, D.G., Akbay, E.A., Kozono, D., Mak, R.H., Makrigiorgos, G.M., Kimmelman, A.C., Wong, K.K. (2014). Image-guided radiotherapy platform using single nodule conditional lung cancer mouse models. Nat Commun. 5:5870.
Herter-Sprie, G.S., Kung, A.L., Wong, K.K. (2013). New cast for a new era: preclinical cancer drug development revisited. J Clin Invest. 123(9):3639-45.
Information from this funding period will not be updated anymore. New research related information is available here.
Mechanism of the »Abscopal Effect« upon Radiation Therapy (RT)
Harnessing CDK4/6 inhibition to promote T cell-mediated anti-tumor immunity

Clinic I of Internal Medicine - CMMC Research Building
CMMC - PI - B 02
CMMC - PI - CAP 16
grit.herter-sprie[at]uk-koeln.de
show more…+49 221 478 89614
Clinic I of Internal Medicine - CMMC Research Building
Robert-Koch-Str. 21
50931 Köln
CMMC Profile Page
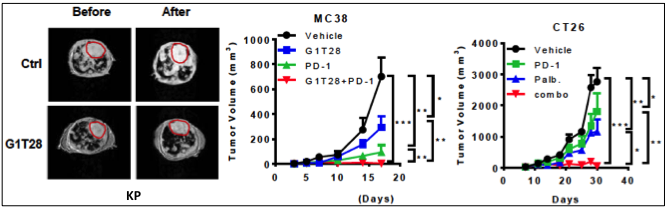
Fig. 1: Dual treatment of CDK4/6 and PD-1 inhibition elicits anti-tumor efficacy. Left panel: Quantification of tumor volume changes by MRI before and after treatment with Trilaciclib of KP mice. Circled areas: heart. Tumor growth curves of MC38 (middle panel) and CT26 (right panel) treated with CDK4/6 inhibitor or PD-1 antibody alone or in combination. (Deng et al., Cancer Discov, 2018).
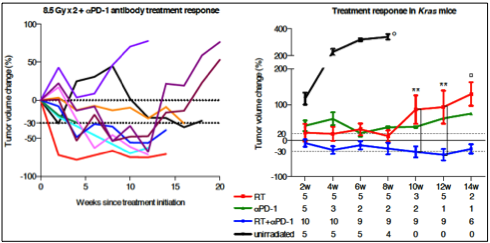
Fig. 2: Blockade of the PD-1/PD-L1 axis potentiates the antitumor efficacy of RTx in Kras-mutant murine NSCLC. Left panel: Tumor volume kinetics after dual treatment with RTx and PD-1 inhibition. Each line represents one mouse. Right panel: Treatment response to RTx, PD-1 inhibition, or a combination of both. Data from unirradiated and RT cohorts were previously published (Herter-Sprie et al., Nat Commun, 2014). Numbers below time points indicate amount of mice evaluated per group. (Herter-Sprie et al., JCI Insight, 2016)