Corneal lymphangiogenesis and activated myeloid cells are important mediators of corneal allograft rejection. Recently, we could also demonstrate an unexpected beneficial effect of immunomodulatory myeloid cells on the resolution of corneal inflammation. The aim of this project was to further characterize the role of myeloid cells in anti-inflammatory lymphangiogenesis in order to develop strategies to resolve corneal inflammation and thereby regain corneal transparency. We wanted gain deeper insight into the interplay between pro(lymph)angiogenic signals, myeloid cells, and the corneal microenvironment to modulate sight-threatening corneal inflammation.
In the past decade we could show that myeloid cells, especially macrophages and dendritic cells, and pathologic corneal lymphatic vessels significantly increase the risk of corneal allograft rejection and developed successful strategies to reduce lymphangiogenesis to achieve a better outcome after transplantation. On the other hand, beneficial, physiological functions for corneal lymphatic vessels, e.g. in corneal wound healing after tissue damage, had not been demonstrated so far. Furthermore, it was not known whether lymphatic vessels might play a role in the regulation of corneal edema, which is one of the most frequent reasons for loss of corneal transparency. It was also unknown how this putative anti-inflammatory lymphangiogenesis would be mediated. A better knowledge of the interplay between vascular growth signals, myeloid cells and the corneal microenvironment is essential to modulate sight-threatening corneal inflammation and prevent corneal blindness.
We could recently show an important role of anti-inflammatory, IL-10 polarized macrophages expressing VEGF-C on the resolution of corneal inflammation (1). Corneal injury in IL-10-deficient mice resulted in less VEGF-C expression, decreased corneal lymphangiogenesis, but more severe and prolonged inflammation. On the other hand, local treatment with IL-10 promoted lymphangiogenesis and faster egress of macrophages from injured corneas. This strongly suggests the hypothesis of a novel therapeutically applicable role of isolated corneal lymphangiogenesis driven by immune modulatory myeloid cells in the resolution of blinding corneal transparency loss caused by inflammation and injury.
Using a novel mouse model of perforating corneal injury that causes acute and severe fluid accu-mulation in the cornea, we could recently demonstrate that corneal lymphatics transiently invade the cornea and regulate the resolution of corneal edema (Figure 1). Pharmacological blockade of lymphangiogenesis via VEGFR-3 inhibition resulted in increased corneal thickness due to delayed drainage of corneal edema and prolonged corneal opacification (2). This work provides first evidence that lymphatic vessels play an un-expectedly beneficial role in the regulation of corneal edema and transparency. This might open new treatment options in blinding diseases asso-ciated with corneal edema and transparency loss.
In order to gain deeper insight into the interplay between pro(lymph)angiogenic signals, myeloid cells, and the inflammatory corneal micro-environment, we analysed the effect of local VEGF-A blockade in the inflamed cornea. We could show that depletion of local VEGF-A increased the expression of pro-inflammatory as well as immune regulatory cytokines in the corneal micro-environment without having a systemic effect (3). These results show how important the pro(lymph)angiogenic factor VEGF-A is also in the regulation of the immunological microenvironment.
Activated leukocyte cell adhesion molecule (ALCAM, CD166) is a cell adhesion molecule of the immune-globulin superfamily and has also been implicated in (lymph)angiogenesis. Using a novel antibody against murine ALCAM we could show that blocking ALCAM leads to DC retention without affecting hem-or lymphangiogenesis. Considering that we also detec-ted ALCAM expression in human corneal DCs and lymphatics (Figure 2), our findings identify ALCAM as a potential novel therapeutic target to modulate the interaction of corneal immune cells and lymphatic vessels (4).
Translationally, we hope to establish novel therapeutic approaches to resolve persisting corneal inflammation to support corneal wound healing as well as to establish new strategies to modulate corneal fluid homeostasis to regain corneal transparency.
1. Hos D, Bucher F, Regenfuss B, Dreisow ML, Bock F, Heindl LM, Eming SA, Cursiefen C. IL-10 indirectly regulates corneal lymphangiogenesis and resolution of inflammation via macrophages. Am J Pathol. (2016);186:159-71.
2. Hos D, Bukowiecki A, Horstmann J, Bock F, Bucher F, Heindl LM, Siebelmann S, Steven P, Dana R, Eming SA, Cursiefen C. Transient ingrowth of lymphatic vessels into the physiologically avascular cornea regulates corneal edema and transparency. Sci Rep. 2017 Aug 3;7(1):7227.
3. Salabarria AC, Braun G, Heykants M, Koch M, Reuten R, Mahabir E, Cursiefen C, Bock F. Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am J Transplant. 2019 Mar 1. [Epub ahead of print]
4. Willrodt AH, Salabarria AC, Schineis P, Ignatova D, Hunter MC, Vranova M, Golding-Ochsenbein AM, Sigmund E, Romagna A, Strassberger V, Fabbi M, Ferrini S, Cursiefen C, Neri D, Guenova E, Bock F, Halin C. ALCAM mediates DC migration through afferent lymphatics and promotes allospecific immune reactions. Front Immunol. 2019 Apr 12;10:759.
5. Kiesewetter A, Cursiefen C, Eming SA, Hos D. Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis. Sci Rep. 2019 Jan 22;9(1):308.
6. Horstmann J, Schulz-Hildebrandt H, Bock F, Siebelmann S, Lankenau E, Hüttmann G, Steven P, Cursiefen C. Label-free in vivo imaging of corneal lymphatic vessels using microscopic optical coherence tomography. Invest Ophthalmol Vis Sci. 2017 Nov 1;58(13):5880-5886
7. Büttner C, Clahsen T, Regenfuss B, Dreisow ML, Steiber Z, Bock F, Reis A, Cursiefen C.Tyrosinase is a novel endogenous regulator of developmental and inflammatory lymphangiogenesis. Am J Pathol. 2019 Feb;189(2):440-448
8. Reuer T, Schneider AC, Cakir B, Bühler AD, Walz JM, Lapp T, Lange C, Agostini H, Schlunck G, Cursiefen C, Reinhard T, Bock F, Stahl A. Semaphorin 3F modulates corneal lymphangiogenesis and promotes corneal graft survival. Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):5277-5284
Bachmann, B., Handel, A., Siebelmann, S., Matthaei, M., and Cursiefen, C. (2019). Mini-Descemet Membrane Endothelial Keratoplasty for the Early Treatment of Acute Corneal Hydrops in Keratoconus. Cornea 38, 1043-8.
Cursiefen, C., Cordeiro, F., Cunha-Vaz, J., Wheeler-Schilling, T., Scholl, H.P.N., and Board, E.V.I.S. (2019). Unmet Needs in Ophthalmology: A European Vision Institute-Consensus Roadmap 2019-2025. Ophthalmic Res10.1159/000501374, 1-11.
Hos, D., Le, V.N.H., Hellmich, M., Siebelmann, S., Roters, S., Bachmann, B.O., and Cursiefen, C. (2019). Risk of Corneal Graft Rejection After High-risk Keratoplasty Following Fine-needle Vessel Coagulation of Corneal Neovascularization Combined With Bevacizumab: A Pilot Study. Transplant Direct 5, e452.
Hos, D., Schaub, F., and Cursiefen, C. (2019). Does anterior chamber-associated immune deviation (ACAID) play a role in posterior lamellar keratoplasty? Case report of a splenectomized patient. BMC Ophthalmol 19, 100.
Kiesewetter, A., Cursiefen, C., Eming, S.A., and Hos, D. (2019). Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis. Sci Rep 9, 308.
Salabarria, A.C., Braun, G., Heykants, M., Koch, M., Reuten, R., Mahabir, E., Cursiefen, C., and Bock, F. (2019). Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am J Transplant10.1111/ajt.15331.
Schaub, F., Bachmann, B.O., Cursiefen, C., and Hos, D. (2019). Midterm follow-up of immune reactions after Descemet membrane endothelial keratoplasty (DMEK). Graefes Arch Clin Exp Ophthalmol 257, 1811-2.
Schrittenlocher, S., Bachmann, B., and Cursiefen, C. (2019). Impact of donor tissue diameter on postoperative central endothelial cell density in Descemet Membrane Endothelial Keratoplasty. Acta Ophthalmol 97, e618-e22.
Hos, D., Matthaei, M., Bock, F., Maruyama, K., Notara, M., Clahsen, T., Hou, Y., Le, V.N.H., Salabarria, A.C., Horstmann, J., Bachmann, B.O., and Cursiefen, C. (2019). Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog Retin Eye Res10.1016/j.preteyeres.2019.07.001.
Salabarria, A.C., Braun, G., Heykants, M., Koch, M., Reuten, R., Mahabir, E., Cursiefen, C., and Bock, F. (2019). Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am J Transplant10.1111/ajt.15331.
Willrodt, A.H., Salabarria, A.C., Schineis, P., Ignatova, D., Hunter, M.C., Vranova, M., Golding-Ochsenbein, A.M., Sigmund, E., Romagna, A., Strassberger, V., Fabbi, M., Ferrini, S., Cursiefen, C., Neri, D., Guenova, E., Bock, F., and Halin, C. (2019). ALCAM Mediates DC Migration Through Afferent Lymphatics and Promotes Allospecific Immune Reactions. Front Immunol 10, 759.
Buttner C, Clahsen T, Regenfuss B, Dreisow ML, Steiber Z, Bock F, Reis A, and Cursiefen C (2018). Tyrosinase Is a Novel Endogenous Regulator of Developmental and Inflammatory Lymphangiogenesis. Am J Pathol 10.1016/j.ajpath.2018.10.014. in 2019
Hou Y, Le VNH, Toth G, Siebelmann S, Horstmann J, Gabriel T, Bock F, and Cursiefen C (2018). UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am J Transplant 10.1111/ajt.14874.
Le VNH, Hou Y, Horstmann J, Bock F, and Cursiefen C (2018). Novel Method to Detect Corneal Lymphatic Vessels In Vivo by Intrastromal Injection of Fluorescein. Cornea 37, 267-271.
Le VNH, Schneider AC, Scholz R, Bock F, and Cursiefen C (2018). Fine Needle-Diathermy Regresses Pathological Corneal (Lymph)Angiogenesis and Promotes High-Risk Corneal Transplant Survival. Sci Rep 8, 5707.
Notara M, Behboudifard S, Kluth MA, Masslo C, Ganss C, Frank MH, Schumacher B, and Cursiefen C (2018). UV light-blocking contact lenses protect against short-term UVB-induced limbal stem cell niche damage and inflammation. Sci Rep 8, 12564.
Notara M, Lentzsch A, Coroneo M, and Cursiefen C (2018). The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int 2018, 8620172.
Reuer T, Schneider AC, Cakir B, Buhler AD, Walz JM, Lapp T, Lange C, Agostini H, Schlunck G, Cursiefen C, Reinhard T, Bock F, and Stahl A (2018). Semaphorin 3F Modulates Corneal Lymphangiogenesis and Promotes Corneal Graft Survival. Invest Ophthalmol Vis Sci 59, 5277-5284.
Schrittenlocher S, Bachmann B, and Cursiefen C (2018). Impact of donor tissue diameter on postoperative central endothelial cell density in Descemet Membrane Endothelial Keratoplasty. Acta Ophthalmol 10.1111/aos.13943.
Schrittenlocher S, Schaub F, Hos D, Siebelmann S, Cursiefen C, and Bachmann B (2018). Evolution of Consecutive Descemet Membrane Endothelial Keratoplasty Outcomes Throughout a 5-Year Period Performed by Two Experienced Surgeons. Am J Ophthalmol 190, 171-178.
Mor JM, Koch KR, Kakkassery V, Cursiefen C, and Heindl LM (2018). [New treatment options for iridociliary tumors]. Ophthalmologe 10.1007/s00347-018-0825-7.
Bock F, and Cursiefen C (2017). Anti(lymph)angiogenic Strategies to Improve Corneal Graft Survival. Klinische Monatsblatter Fur Augenheilkunde 234, 674-8.
Bukowiecki A, Hos D, Cursiefen C, and Eming SA (2017). Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int J Mol Sci 18.
Horstmann J, Schulz-Hildebrandt H, Bock F, Siebelmann S, Lankenau E, Huttmann G, Steven P, and Cursiefen C (2017). Label-Free In Vivo Imaging of Corneal Lymphatic Vessels Using Microscopic Optical Coherence Tomography. Invest Ophthalmol Vis Sci 58, 5880-6.
Hos D, Bukowiecki A, Horstmann J, Bock F, Bucher F, Heindl LM, Siebelmann S, Steven P, Dana R, Eming SA, and Cursiefen C (2017). Transient Ingrowth of Lymphatic Vessels into the Physiologically Avascular Cornea Regulates Corneal Edema and Transparency. Sci Rep 7, 7227.
Hos NJ, Ganesan R, Gutierrez S, Hos D, Klimek J, Abdullah Z, Kronke M, and Robinson N (2017c). Type I interferon enhances necroptosis of Salmonella Typhimurium-infected macrophages by impairing antioxidative stress responses. J Cell Biol 216, 4107-21.
Hou Y, Le VNH, Clahsen T, Schneider AC, Bock F, and Cursiefen C (2017). Photodynamic Therapy Leads to Time-Dependent Regression of Pathologic Corneal (Lymph) Angiogenesis and Promotes High-Risk Corneal Allograft Survival. Invest Ophthalmol Vis Sci 58, 5862-9.
Hou Y, Le VNH, Clahsen T, Schneider AC, Bock F, and Cursiefen C (2017). Photodynamic Therapy Leads to Time-Dependent Regression of Pathologic Corneal (Lymph) Angiogenesis and Promotes High-Risk Corneal Allograft Survival. Invest Ophthalmol Vis Sci 58, 5862-9.
Le VNH, Hou Y, Horstmann J, Bock F, and Cursiefen C (2017). Novel Method to Detect Corneal Lymphatic Vessels In Vivo by Intrastromal Injection of Fluorescein. Cornea 10.1097/ICO.0000000000001444.
Information from this funding period will not be updated anymore. New research related information is available here.
Role and therapeutic potential of autophagy-related mechanisms in UV-induced blinding ocular stem cell disorders (Pterygium)
Pending
The contribution of myeloid cells to corneal neovascular disease

Clinic of General Ophthalmology
CMMC - PI - B 04
Executive Board Member
claus.cursiefen[at]uk-koeln.de
show more…+49 221 478 4300
+49 221 478 97295
Clinic of General Ophthalmology
Kerpener Str. 62
50937 Cologne
https://augenklinik.uk-koeln.de/forschung/arbeitsgruppen-labore/cornea-lab/

Clinic of General Ophthalmology
CMMC - PI - assoc. RG 04
show more…+49 221 478 97789
+49 221 478 86465
Clinic of General Ophthalmology
Kerpener Str. 62
50924 Cologne

Clinic of General Ophthalmology
CMMC - Co-PI - B 04
CMMC - PI - CAP 11
+49 221 478 98896
+49 221 478 32400
Clinic of General Ophthalmology
Kerpener Str. 62
50924 Cologne
Maria Notara (Postdoc)
Thomas Clahsen (Postdoc)
Yanhong Hou (doctoral student)
Hung Le (doctoral student)
Alfrun Schönberg (doctoral student)
Gabriele Braun (technician)
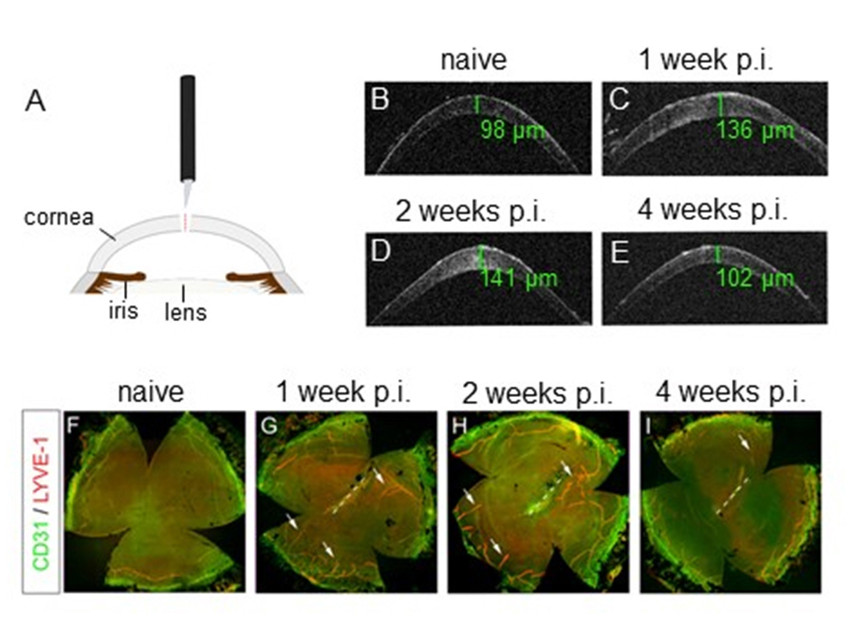
Figure 1: Perforating corneal injury induces transient edema and isolated lymphangiogenesis without hemangio-genesis. (A) Model of perforating corneal injury. (B–E) In vivo optical coherence tomography scans demonstrate a transient increase of corneal thickness, green values: central corneal thickness. (F–I) Corneal whole mounts stained with LYVE-1 (red) and CD31 (green); LYVE-1high/CD31low lymphatic vessels (arrows) growing towards the central cornea are detectable 1 week after injury and persist until 2 weeks after injury. Afterwards, lymphatic vessels regress and are comparable to uninjured corneas after 4 weeks. In contrast to lymphatic vessels, no significant ingrowth of LYVE-1neg/CD31high blood vessels is detectable; p.i.: post injury. Modified from (2).