The identification of primary genetic hits and the target cell population is of great benefit for an early detection of malignancies and a better prediction of cancer growth. The important finding that stem cells give rise to tumours in a variety of tissues is highly relevant for improving current concepts for treatment and for designing preventive therapies. The project aims to decipher stem cell-specific mechanisms driving tumour initiation and to explore targeting disease-initiating events as a promising therapeutic strategy.
Both leukaemia and solid tumours show substantial heterogeneity in their cellular morphology, proliferative index, genetic lesions and therapeutic response. The molecular and cellular mechanisms underlying tumour heterogeneity remain central questions in the field of cancer biology and therapy. It is widely accepted that the pattern of dominant mutations as well as the ‘cell-of-origin’ determine phenotype, growth potential and malignant progression of a distinct tumour type. Cells giving rise to tumours acquire the first genetic hits that eventually enable cells escaping regulatory mechanisms of normal growth and differentiation within a giving tissue.
Obviously, identification of these crucial target cell populations facilitates unravelling significant tumour-initiating mechanisms, rendering the possibility of earlier detection of malignancies and better prediction of tumour behaviour. Importantly, previous findings convincingly showed that tissue stem and progenitor cells give rise to a range of solid tumours, including epidermal cancer. These results highlight the necessity to investigate relevant basic mechanisms underlying the process of stem cell-driven tumour formation and malignant progression.
Previous work identified hair follicle stem cells as ‘cell-of-origin’ for a variety of different types of epidermal cancer. To address the question why particularly hair follicle stem cells give rise to tumours, molecular and cellular alterations within the stem cell compartment preceding tumour development are under investigation. Initial studies revealed that hair follicle stem cells are characterised by faster DNA repair activity when compared to other epidermal cells. This involved non-homologous end joining (NHEJ) and the stimulation of the key protein DNA-PK (DNA-dependent protein kinase). Thus, although this hair follicle stem cell-specific DNA damage response mechanism evolved, NHEJ is an error prone repair mechanism potentially resulting in accumulation of harmful mutations and thus promoting stem cell-driven tumour formation.
Further, our own studies demonstrate that stem cells feature specific cell death modalities and that subpopulations of stem cells are specifically protected from undergoing apoptosis. Currently, our research aims to identify the detailed molecular mechanisms mediating a distinct stem cell death response during tissue homeostasis and regeneration. Although our experimental work is still on-going, the data indicate that defined mechanisms evolved to maintain stem cells and to secure tissue integrity.
However, protection of tissue stem cells from undergoing apoptosis could lead to the accumulation of damaged stem cells with profound implications for the process of cancer development. This project further addresses the important issue of long-term consequences of defective regulation of stem cell safeguard mechanisms and analysis the contribution to tumour initiation.
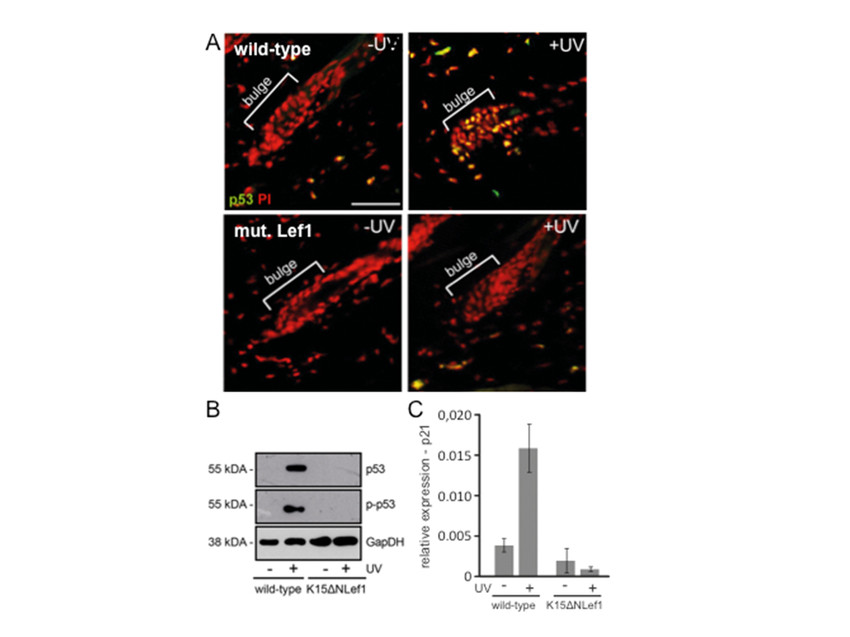
Functional p53 is involved in the regulation of multiple cellular processes, including cell cycle arrest, apoptosis, cellular senescence and DNA damage response. The tumour suppressor gene TP53 is the most frequently mutated gene in human cancer, including skin cancer, and p53 activity is altered in approximately 50% of human malignancies. Interestingly, hair follicle stem cells show accelerated p53 activation in response to tissue damage by irradiation. This essential response mechanism is blocked by oncogenic stress, e.g. expression of a mutant form of the transcription factor Lef1 (Figure 1).
The project aims to unravel the underlying mechanisms of an accelerated p53 response detected in hair follicle stem cells. Furthermore, consequences of a defective p53 stem cell response mechanism for the process of epidermal cancer development are investigated.
Cancers lacking p53 mutations often display inactivated wild-type (wt) p53 through modulation of protein interactions (e.g. MDM2/MDM4) resulting in p53 protein degradation. Interestingly, our data reveal that post-translational p53 protein regulation is defective in mutant Lef1 stem cells. Currently, we are testing the possibility restoring normal p53 function in tumour-initiating stem cells and explore consequences for the process of disease formation.
Our recent work shed light on the biological function and control mechanisms of p53 activity in the so far unexplored context of stem cell-driven cancer. In addition, our work will result in novel insights into upstream regulatory pathways leading to a block of p53 function as well as into stem cell-specific mechanisms of p53.
Until now it is not sufficiently known how p53 integrates with other oncogenic pathways, including β-catenin/Lef1 signaling. The novel results should be of great importance for a concept of restoration of p53 function in skin cancer as a sound option for therapy. Moreover, better understanding the role of stem cell-specific gatekeeper functions for tumor initiation and cancer stem cell activity in general would be highly beneficial for improving cancer treatment strategies.
1. Singh, K., Camera, E., Krug, L., Basu, A., Pandey, R.K., Munir, S., Wlaschek, M., Kochanek, S., Schorpp-Kistner, M., Picardo, M., Angel, P., Niemann, C., Maity, P., Scharffetter-Kochanek, K. (2018). JunB defines functional and structural integrity of the epidermo-pilosebaceous unit in the skin. Nature Communications 2018: 9, 3425.
2. Charcón-Martinez, C.A., Klose, M., Niemann, C., Glauche, I., Wickström, S.A. (2017). Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. EMBO J. 36, 679-89.
3. Petersson, M.*, Reuter, K.*, Brylka, H., Schettina, P., Kraus, A., Niemann, C. (2015). Interfering with stem cell-specific gatekeeper functions controls tumor initiation and malignant progression of skin tumors. Nature Communications 6, 5874.
4. Frances, D., Sharma, N., Pofahl, R., Maneck, M., Behrendt, K., Reuter, K., Krieg, T., Klein, C.A., Haase, I., Niemann, C. (2015). A role for Rac1 activity in malignant progression of sebaceous skin tumours. Oncogene 34, 5505-12.
5. Petersson, M., Niemann, C. (2012). Stem cell dynamics and heterogeneity : Implications for epidermal regeneration and skin cancer. Curr Med Chem. 19, 5948-92.
Singh K, Camera E, Krug L, Basu A, Pandey RK, Munir S, Wlaschek M, Kochanek S, Schorpp-Kistner M, Picardo M, Angel P, Niemann C, Maity P, and Scharffetter-Kochanek K (2018). JunB defines functional and structural integrity of the epidermo-pilosebaceous unit in the skin. Nat Commun 9, 3425.
Chacon-Martinez CA, Klose M, Niemann C, Glauche I, and Wickstrom SA (2017). Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. EMBO J 36, 151-64.
Information from this funding period will not be updated anymore. New research related information is available here.

Center for Molecular Medicine Cologne - CMMC Research Building
CMMC - PI - assoc. RG 14
IPMM Program - Scientific Coordinator I CMMC Tissue Embedding and Histology & Microscopy - Head
+49 221 478 89511
+49 221 478 86737
Center for Molecular Medicine Cologne - CMMC Research Building
Robert-Koch-Str. 21
50931 Cologne
Marcel Drews (PhD student)
Anna Geueke (PhD student)
Gökçen Gözüm (PhD student)
Giada Mantellato (PhD student)
Florian Küster (Master student)
Jan-Marc Leonhard (technician)
Melanie Nelles (technician)
Peter Schettina (technician)