Glucocorticoids have a significant impact on the occurrence and the clinical outcome of infections caused by intracellular pathogens, yet their effect on the human defense response is incompletely understood. Here, we investigate how glucocorticoids regulate macrophage host defense pathways against intracellular pathogens. In the long-term our work may help reducing infection risks of glucocorticoid therapy for inflammatory diseases and optimizing treatments for inflammatory forms of intracellular infections.
In dermatology - and in almost all other fields of medicine - immunosuppressive drugs, such as glucocorticoids, are widely used to treat auto-immunity, allergies and auto-inflammation. A common problem of these therapies is an increased risk for the development of infections, including those caused by intracellular pathogens, for instance tuberculosis. Nevertheless, overwhelming inflammation as a consequence of the host response against intracellular pathogens can also contribute to immunopathology and tissue damage. Examples include inflammatory forms of tuberculosis and reactional states in leprosy. In these situations adjuvant immune-suppression, e.g. by glucocorticoids, is beneficial for the patients. Meanwhile, it is incompletely understood how immune-suppressive therapies impact antimicrobial host responses. In this project, we are investigating the effect of glucocorticoids on the immune response of human macrophages against intracellular pathogens, using mycobacteria as a model.
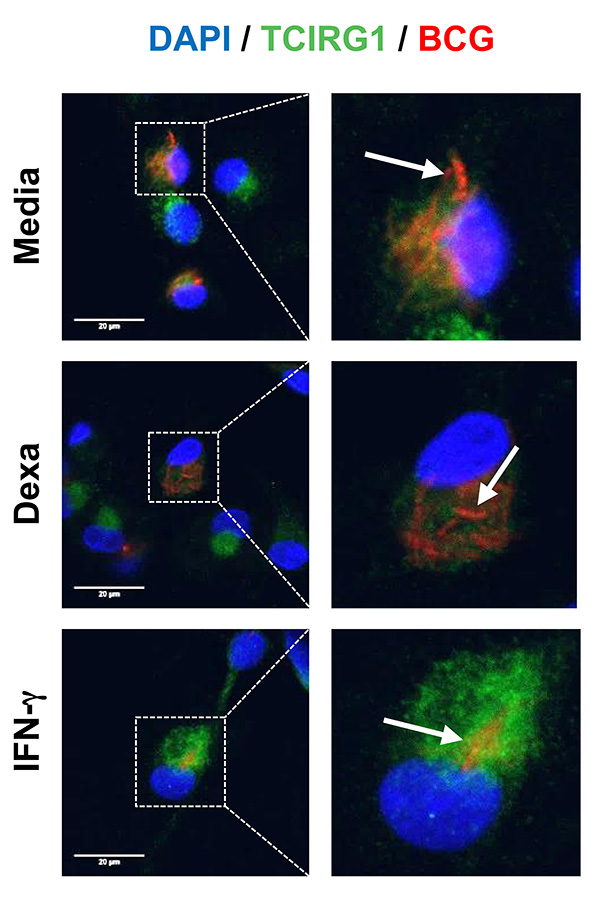
Macrophages are commonly infected by intracellular pathogens, such as mycobacteria. The ability of macrophages to kill intracellular pathogens is crucial for the outcome of human infections. We asked whether glucocorticoids directly regulate the macrophage anti-mycobacterial host response. By comparing primary human macrophages stimulated with glucocorticoids or IFN-γ, a key activator of macrophage host defense, we provided evidence that glucocorticoids induce cathelicidin, an antimicrobial peptide that is pivotal to the host response against mycobacteria in human macrophages. Induction of cathelicidin by glucocorticoids was independent of the intracellular vitamin D metabolism, therefore different from the IFN-γ-mediated induction of cathelicidin. Surprisingly, despite inducing cathelicidin, glucocorticoids failed to trigger antimicrobial activity. To investigate the underlying reasons we performed whole genome mRNA expression arrays of glucocorticoid- vs. IFN-γ-stimulated macrophages. Using weighted gene coexpression network analyses (WGCNA), a systems biology approach, we identified a network of coexpressed genes linked to key macrophages host defense pathways, including “autophagy”, “phagosome maturation” and “lytic vacuole/lysosome”, that was inversely correlated with glucocorticoid stimulation and associated with IFN-γ treatment. Furthermore, we identified the vacuolar H+-ATPase V0 subunit a3 (TCIRG1), a central host defense gene against mycobacteria, as the top hub gene in this module. Subsequently, we showed in detail that IFN-γ triggers expression and phagolysosome recruitment of TCIRG1, as well as lysosome acidification, which was dependent on autophagy induction. In contrast, the inability of glucocorticoids to promote antimicrobial activity is linked to a failure to induce TCIRG1, phagolysosome recruitment and lysosome acidification (Figure 1). In summary, these data show that glucocorticoids dissociate anti-mycobacterial pathways in human macrophages. Importantly, glucocorticoid induction of cathelicidin is not sufficient to trigger antimicrobial activity in macrophages. Instead, the ability of IFN-γ to induce autophagy, as well as phagolysosome maturation and acidification, which are not induced by glucocorticoids, is crucial for human host defense.
In the last years the tyrosine kinase inhibitor imatinib has emerged as a host-directed drug for tuberculosis and other infections. In human macrophages imatinib was shown to induce the vacuolar H+-ATPase, leading to lysosome acidification and antimicrobial activity against M. tuberculosis. Therefore, we investigated whether imatinib could overcome the failure of glucocorticoids to promote lysosome acidification. We found that imatinib in combination with glucocorticoids promotes lysosome acidification and induces mycobacterial growth restriction in human macrophages, without diminishing the anti-inflammatory effects of glucocorticoids. This suggests that imatinib treatment is a possible host-directed therapy in patients receiving glucocorticoids for treatment of inflammatory diseases. Imatinib might be in particular useful in the context of immune reconstitution inflammatory syndrome (IRIS), which occurs during the initiation of HIV therapy and is treated by glucocorticoids.
Recent evidence by other research groups suggests that glucocorticoids enhance central aspects of the innate immune response. In this regard, glucocorticoids were shown to enhance expression of TLR2 in various cell types, implying the possibility that glucocorticoids act to ready innate host defense. Given a central role of TLR2 in activating macrophage host defense against intracellular pathogens, we studied the effect of glucocorticoids on expression of TLR2 in primary human macrophages, finding that glucocorticoids upregulated TLR2 mRNA expression and cell surface expression. In ongoing work we are studying the effect of glucocorticoids on TLR2 signaling and the functional consequences on TLR2-induced inflammatory and antimicrobial responses.
The long-term goal of our studies is to understand the effect of immune-suppressive therapies on the human antimicrobial host response against intracellular infection and on the underlying mechanisms. We hope that a better understanding of the mechanisms, by which immune-suppressive drugs regulate the human host response, will allow to therapeutically manipulating pathways in a way to take advantage of the anti-inflammatory effects to restrict tissue damage, while simultaneously sustaining or even enhancing antimicrobial responses.
Steiger, J., Stephan, A., Inkeles, M.S., Realegeno, S., Bruns, H., Kröll, P., de Castro Kroner, J., Sommer, A., Batinica, M., Pitzler, L., Kalscheuer, R., Hartmann, P., Plum, G., Stenger, S., Pellegrini, M., Brachvogel, B., Modlin, R.L., and Fabri, M. (2016).
Imatinib triggers phagolysosome acidification and antimicrobial activity against BCG in glucocorticoid-treated human macrophages. J Immunol, 197, 222-32.
Bruns, H., Büttner, M., Fabri, M., Mougiakakos, D., Bittenbring, JT., Hoffmann, M.H., Beier, F., Pasemann, S., Jitschin, R., Hofmann, A.D., Neumann, F., Daniel, C., Maurberger, A., Kempkes, B., Amann, K., Mackensen, A., Gerbitz, A. (2015).
Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high grade B-cell lymphoma. Sci. Transl. Med, 7, 282ra47.
Information from this funding period will not be updated anymore. New research related information is available here.

Clinic of Dermatology and Venereology
CMMC - Co-PI - C 04
show more…+49 221 478 82269
+49 221 478 5949
Clinic of Dermatology and Venereology
Kerpener Str. 62
50931 Cologne
Julia Steiger (Technician)
Juliana de Castro Kroner (PhD student)
Alexander Stephan (PhD student)
Lisa Marienfeld (MD student)
Philip Kröll (MD student)