Increased activity of the type II transmembrane protease matriptase 1 (ST14) is implicated in multiple epithelial malignancies, while other data point to tumor-suppressive roles. However, the molecular basis of these functions and ST14’s double face remains elusive. We have screened small molecule libraries for components affecting the mild malignancy caused by gain of ST14 function in the epidermis of zebrafish embryos, thereby identifying both oncogenic and tumor-suppressive pathways downstream of ST14.
ST14 (Suppression of Tumorigenicity-14) is a cell surface-associated protease that reshapes its microenvironment by degrading ECM proteins, while cleaving and activating signaling molecules like growth factors and their receptors. This pericellular proteolysis is crucial for multiple processes of epithelial development, homeostasis and regeneration.
On the other hand, increased pericellular proteolysis is critically involved in tumor growth and invasiveness, and deregulation of matriptases has been implicated in a variety of epithelial cancers (List, 2009). On the other hand, tumor-suppressive functions of ST14 have been revealed (Kosa et al., 2012), as also reflected by its name. About a dozen ST14 target proteins have been identified and characterized, mainly using in vitro systems.
This list is far from complete, and the in vivo relevance of identified targets, in particular in the context of carcinogenesis, remains largely elusive, although better knowledge would greatly facilitate the development of targeted anti-carcinogenic therapies.
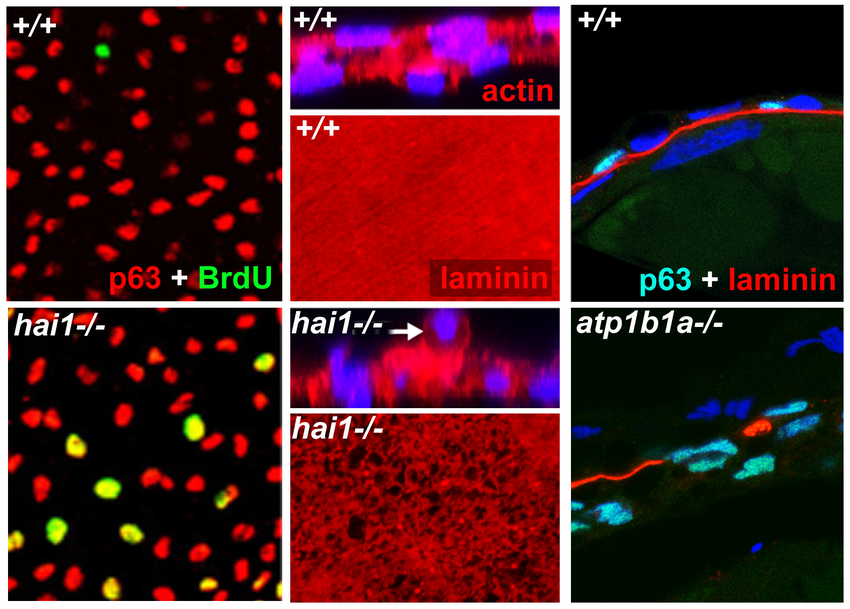
We have identified zebrafish mutants in the matriptase inhibitor Hai1, which due to increased ST14 activity display compromised epithelial integrity of the embryonic epidermis, keratinocyte hyperproliferation (Carney et al., 2007) and partial basement membrane degradation, signs of early stage carcinogenesis. However, keratinocytes usually do not invade the underlying dermis, but are extruded apically, which has a tumor-suppressive effect (Figure 1), possibly underlying the progressive healing of the skin defects of hai1 mutants. In contrast, zebrafish mutants in ATP1b1a, a Na/K-ATPase -subunit, display stronger and progressive epidermal malignancy, with transformed keratinocytes extruding basally and invading the dermis and, possibly, other tissues (Figure 1).
Carcinogenesis is completely rescued upon incubation of atp1b1a mutant embryos in an isotonic, rather than the natural hypotonic environment, pointing to hypotonic stress as a thus far unrecognized tumor risk factor that is likely to be also relevant during human carcinogesis (Hatzold et al., 2016).
In light of its pivotal role during carcinogenesis, numerous efforts have been undertaken to design ST14 inhibitors (e.g. Quimbar et al., 2013). However, they failed to rescue the defects of hai1 mutant embryos (unpublished data), indicating that they have no or little effects when applied in vivo. We carried out an unbiased chemical library screen to identify effectors of ST14-dependent carcinogenesis at any level upstream, downstream or in parallel to ST14.
Since most of the identified chemicals are well annotated, with known targets, this also allowed us to elucidate pathways downstream of ST14 mediating carcinogenesis or tumor suppression. Crucial identified pathway components are the epidermal growth factor receptor, mTORC1, PPARγ and phospholipase D. Strikingly, the latter has both an oncogenic (via mTORC1) and a tumor-suppressive (apical cell extrusion-promoting) effect, with PLD inhibition leading to a transient improvement, but long-term worsening of epidermal malignancy in hai1 mutant embryos, in line with the aforementioned double face of ST14 itself (unpublished data).
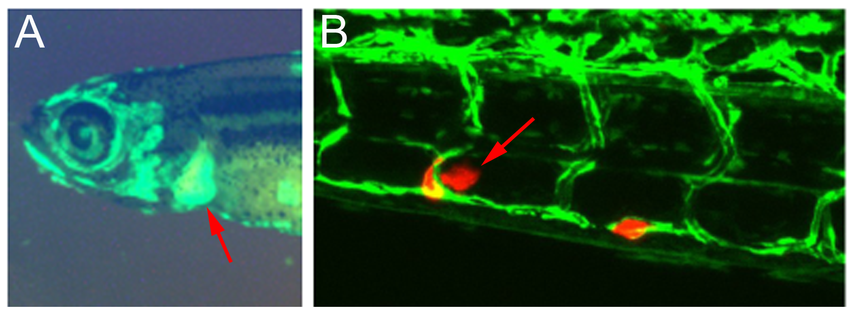
Testing small compounds effective in hai1 mutants also in zebrafish atp1b1a mutants (see above), we found that some oncogenic components are shared by the two epidermal malignancy systems, while others are system-specific. In the future, we will also test identified components singly and in combination in other zebrafish carcinogenesis systems we have established in the laboratory to study adult carcinogenesis and metastasis (invasiveness via the blood vessel system; Figure 2). Furthermore, identified drugs and hypotonic stress as a potential tumor-promoting factor are tested in mammalian carcinogenesis systems, such as cultured transformed human keratinocytes and human non-small lung cancer cells (NSCLC).
The ultimate goal of the project is to tailor improved human cancer therapies, treating carcinomas with combinations of identified small molecules to specifically block oncogenic pathways, while leaving tumor-suppressive pathways unaffected. In addition, isotonic treatments might help to treat carcinomas in which cancer cells are exposed to a hypotonic environment, as in open lung cancers (when cells are exposed to the airway surface fluid) or esophageal cancers (when cells are exposed to saliva).
Carney, T.J., von der Hardt, S:, Sonntag, C., Amsterdam, A., Topczewski, J., Hopkins, N. and Hammerschmidt, M. (2007). Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 134, 3461-71.
Hatzold, J., Beleggia, F., Herzig, H., Altmüller, J., Nürnberg, P., Bloch, W., Wollnik, B. and Hammerschmidt, M. (2016). Tumor suppression in basal keratinocytes via dual non-cell autonomous functions of a Na,K-ATPase beta subunit. Elife 5, e14277.
Kosa, P., Szabo, R., Molinolo, A.A. and Bugge, T.H. (2012). Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis. Oncogene 31, 3679-95.
List, K. (2009). Matriptase: a culprit in cancer? Future Oncol 5, 97-104
Qumibar, P., Malik, U., Sommerhoff, C.P., Kaas, Q., Chan, L.Y., Huang, Y.H., Grundhuber, M., Dunse, K., Craik, D.J., Anderson, M.A. and Daly, N.L. (2013). High-affinity cyclic peptide matriptase inhibitors. J. Biol. Chem. 288, 13885-96
Information from this funding period will not be updated anymore. New research related information is available here.

Institute of Zoology - Biocenter Cologne
CMMC - PI - C 06
Executive Board Member
+49 221 470 5665
+49 221 470 5184
Institute of Zoology - Biocenter Cologne
Zülpicher Str. 47b
50674 Cologne
Hans-Martin Pogoda (Senior Scientist)
Julia Hatzold (PostDoc)
Joy Armistead (PostDoc)
Cornelia Stein (PostDoc)
Erica Benard (Postdoc)
Heiko Löhr (PostDoc)
Daniela Welcker (PostDoc)
Manuel Metzger (PhD student)
Philip Reinoss (PhD student)
Heike Wessendorf (Technician)
Iris Quinkertz-Riedl (Technician)
Evelin Fahle (Technician)
Christel Schenkel (Technician)