The power of anti-cancer therapies is often compromised by the fact that targeted cells, processes and/or molecules can display a combination of oncogenic and tumor-suppressive effects. Our work has unravelled a similar double face for Matriptase 1, also named ST14, a type II transmembrane serine protease, and its downstream mediators epidermal growth factor receptor (EGFR) and phospholipase D (PLD). In zebrafish embryos lacking the cognate ST14 inhibitor Hai1, the hyper-stimulated ST14 – EGFR – PLD pathway promotes both mTORC1-mediated epidermal hyperplasia (oncogenic) and sphingosine-1-phosphate (S1P)-mediated epidermal cell loss (tumor-suppressive). The latter involves entosis of preneoplastic basal keratinocytes by neighboring surface keratinocytes and the subsequent apical extrusion of these cell complexes. Accordingly, combined treatment with the PLD inhibitor FIPI to block the shared signalling pathway and S1P to specifically reactivate the tumor-suppressive branch leads to a perfect rescue and survival of otherwise lethal zebrafish hai1 null mutants. This treatment regime needs to be tested in mammalian carcinoma systems. In addition, the epigenetic basis of the heterogeneity among basal keratinocytes of zebrafish hai1 mutants, and the genetic control of the cell competition, entosis and apical cell extrusion processes leading to the preferential extrusion of preneoplastic basal cells need to be further dissected and characterized.
ST14 (Suppression of Tumorigenicity-14) is an epithelia-specific cell surface-associated protease that reshapes its microenvironment by degrading ECM proteins, while cleaving and activating signaling molecules like growth factors and their receptors. This pericellular proteolysis is crucial for multiple processes of epithelial development, homeostasis and regeneration. Pericellular proteolysis is also critically involved in tumor growth and invasiveness, and upregulation of ST14 has been implicated in a variety of epithelial cancers, while on the other hand, tumor-suppressive functions of ST14 have been revealed (Tanabe and List, 2017). About a dozen ST14 target proteins have been identified and characterized, mainly using in vitro systems. However, the in vivo relevance of identified targets, in particular in the context of carcinogenesis, remains largely elusive, although better knowledge would greatly facilitate the development of targeted anti-carcinogenic therapies.
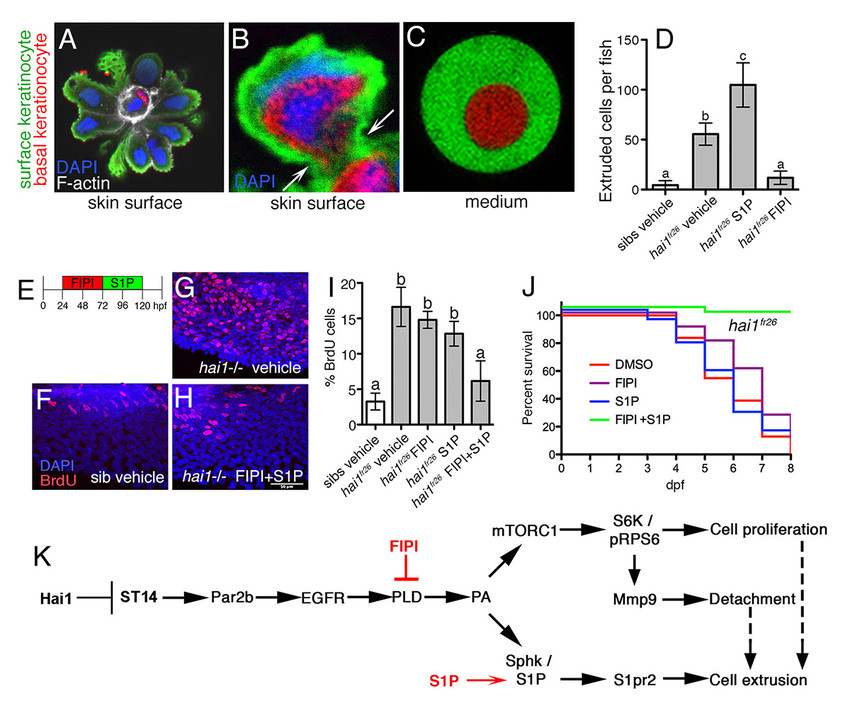
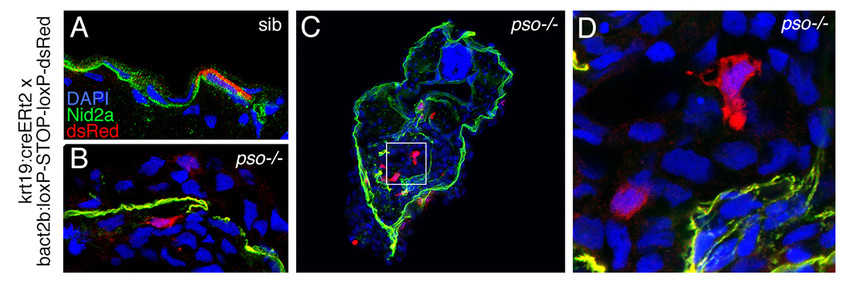
We have identified zebrafish mutants in the ST14 inhibitor Hai1, which due to increased ST14 activity display compromised epithelial integrity of the embryonic epidermis, keratinocyte hyper-proliferation (Carney et al., 2007; Figure 1E-I) and partial basement membrane degradation, signs of early stage carcinogenesis. However, keratinocytes usually do not invade the underlying dermis, but are extruded apically, which has a tumor-suppressive effect (Figure 1A-D), possibly underlying the progressive healing of the skin defects of hypomorphic hai1 mutants. In contrast, basal keratinocytes of zebrafish pso mutants in ATP1b1a, a Na/K-ATPase β-subunit, display stronger ST14-dependent epidermal malignancy, with basal keratinocytes leaving basally and undergoing metastasis (Hatzold et al., 2016 and unpublished results, Figure 2).
To identify effectors of ST14-dependent carcinogenesis, we carried out a systematic search for annotated small compounds that alleviate or worsen the epidermal defects of hypomorphic zebrafish hai1 mutants. Crucial pathway components identified in this manner are the formerly known ST14 targets Par2b and EGFR and, as a novel mediator, phospholipase D (PLD).
Strikingly, the pathway branches at the level of the PLD product phosphatidic acid (PA), which promotes keratinocyte hyperproliferation, invasiveness and thereby oncogenesis via mTORC1 (branch 1), and apical cell extrusion and thereby tumor suppression via sphingosine kinase (Sphk) and its product sphingosine-1-phosphate / S1P (branch 2; Figure 1K). Accordingly, amorphic hai1 mutants, which in contrast to the spontaneously healing hypomorphs are lethal, can only be temporarily rescued by pharmacological inhibition of PLD with FIPI. Later, most likely due to the concomitant blockage of the tumor-suppressive branch, they relapse with epidermal hyperplasia even more severe than in untreated mutant siblings. To circumvent this relapse, we reactivated apical cell extrusion in FIPI-treated mutants via administration of S1P (Figure 1E), resulting in a long-lasting rescue of epidermal hyperplasia and a complete survival of the otherwise lethal mutants (Figure 1F-J) (Armistead et al., in revision). We also discovered how apical cell extrusion, which occurs at the surface of the skin, can be tumor-suppressive, given that it is the basal keratinocytes that hyper-proliferate: before their own extrusion, surface cells selectively engulf underlying preneoplastic basal cells (Figure 1A-C) in a process similar to entosis that was formerly identified in epithelial cancer cell lines but had not been described for carcinoma cells in vivo thus far.
The ultimate goal of the project is to tailor improved human cancer therapies, treating carcinomas with combinations of identified small molecules to specifically block oncogenic pathways, while leaving tumor-suppressive pathways and the according processes (cell-cell competition, entosis, apical cell extrusion) unaffected.
1. Armistead, J., Hatzold, J., van Roye, A., Fahle, E. and Hammerschmidt M. Entosis and apical cell extrusion constitute a tumour suppressive mechanism downstream of Matriptase. J. Cell Biol., in revision
2. Carney, T.J., von der Hardt, S., Sonntag, C., Amsterdam, A., Topczewski, J., Hopkins, N. and Hammerschmidt, M. (2007). Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 134, 3461-71
3. Hatzold, J., Beleggia, F., Herzig, H., Altmüller, J., Nürnberg, P., Bloch, W., Wollnik, B. and Hammerschmidt, M. (2016). Tumor suppression in basal keratinocytes via dual non-cell autonomous functions of a Na,K-ATPase beta subunit. Elife 5, e14277
4. Tanabe, L.M. and List, K. (2017). The role of type II transmembrane serine protease mediated signaling in cancer. FEBS J. 284, 1421-36
Jurkute, N., Leu, C., Pogoda, H.M., Arno, G., Robson, A.G., Nurnberg, G., Altmuller, J., Thiele, H., Motameny, S., Toliat, M.R., Powell, K., Hohne, W., Michaelides, M., Webster, A.R., Moore, A.T., Hammerschmidt, M., Nurnberg, P., Yu-Wai-Man, P., and Votruba, M. (2019). SSBP1 mutations in dominant optic atrophy with variable retinal degeneration. Ann Neurol 86, 368-83.
Janzen E, Mendoza-Ferreira N, Hosseinibarkooie S, Schneider S, Hupperich K, Tschanz T, Grysko V, Riessland M, Hammerschmidt M, Rigo F, Bennett CF, Kye MJ, Torres-Benito L, and Wirth B (2018). CHP1 reduction ameliorates spinal muscular atrophy pathology by restoring calcineurin activity and endocytosis. Brain 10.1093/brain/awy167.
Lohr H, Hess S, Pereira MMA, Reinoss P, Leibold S, Schenkel C, Wunderlich CM, Kloppenburg P, Bruning JC, and Hammerschmidt M (2018). Diet-Induced Growth Is Regulated via Acquired Leptin Resistance and Engages a Pomc-Somatostatin-Growth Hormone Circuit. Cell Rep 23, 1728-1741.
Mendoza-Ferreira N, Coutelier M, Janzen E, Hosseinibarkooie S, Lohr H, Schneider S, Milbradt J, Karakaya M, Riessland M, Pichlo C, Torres-Benito L, Singleton A, Zuchner S, Brice A, Durr A, Hammerschmidt M, Stevanin G, and Wirth B (2018). Biallelic CHP1 mutation causes human autosomal recessive ataxia by impairing NHE1 function. Neurol Genet 4, e209.
Pogoda HM, Riedl-Quinkertz I, Lohr H, Waxman JS, Dale RM, Topczewski J, Schulte-Merker S, and Hammerschmidt M (2018). Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development. Development 145.
van der Ven AT, Kobbe B, Kohl S, Shril S, Pogoda HM, Imhof T, Ityel H, Vivante A, Chen J, Hwang DY, Connaughton DM, Mann N, Widmeier E, Taglienti M, Schmidt JM, Nakayama M, Senguttuvan P, Kumar S, Tasic V, Kehinde EO, Mane SM, Lifton RP, Soliman N, Lu W, Bauer SB, Hammerschmidt M, Wagener R, and Hildebrandt F (2018). A homozygous missense variant in VWA2, encoding an interactor of the Fraser-complex, in a patient with vesicoureteral reflux. PLoS One 13, e0191224.
Richardson R, and Hammerschmidt M (2018). The role of Rho kinase (Rock) in re-epithelialization of adult zebrafish skin wounds. Small GTPases 9, 230-236.
Lessel D, Wu D, Trujillo C, Ramezani T, Lessel I, Alwasiyah MK, Saha B, Hisama FM, Rading K, Goebel I, Schutz P, Speit G, Hogel J, Thiele H, Nurnberg G, Nurnberg P, Hammerschmidt M, Zhu Y, Tong DR, Katz C, Martin GM, Oshima J, Prives C, and Kubisch C (2017). Dysfunction of the MDM2/p53 axis is linked to premature aging. J Clin Invest 127, 3598-608.
Riessland M, Kaczmarek A, Schneider S, Swoboda KJ, Lohr H, Bradler C, Grysko V, Dimitriadi M, Hosseinibarkooie S, Torres-Benito L, Peters M, Upadhyay A, Biglari N, Krober S, Holker I, Garbes L, Gilissen C, Hoischen A, Nurnberg G, Nurnberg P, Walter M, Rigo F, Bennett CF, Kye MJ, Hart AC, Hammerschmidt M, Kloppenburg P, and Wirth B (2017). Neurocalcin Delta Suppression Protects against Spinal Muscular Atrophy in Humans and across Species by Restoring Impaired Endocytosis. Am J Hum Genet 100, 297-315.
Information from this funding period will not be updated anymore. New research related information is available here.
The double faces of ST14, Notch signaling and p53, and the impact of cell competition, entosis and apical cell extrusion during carcinogenesis and tumor suppression

Institute of Zoology - Biocenter Cologne
CMMC - PI - C 06
Executive Board Member
+49 221 470 5665
+49 221 470 5184
Institute of Zoology - Biocenter Cologne
Zülpicher Str. 47b
50674 Cologne
Hans-Martin Pogoda (Senior Scientist)
Julia Hatzold (PostDoc)
Joy Armistead (PostDoc)
Erica Benard (PostDoc)
Daniela Welcker (PostDoc)
Ismail Küçükaylak (PhD Student)
Indra Möllenkotte (PostDoc)
Madhuri Puvvada (PhD Student)
Philip Reinoss (PhD Student)
Heike Wessendorf (Technician)
Iris Quinkertz-Riedl (Technician)
Evelin Fahle (Technician)
Christel Schenkel (Technician)