Our aim was to apply our interdisciplinary expertise of genetics, biochemistry and structural biology to underpin how functional centrosomes are built and how do they control cell proliferation, differentiatuion and organ development. To accomplish the overall goal, we solved the crystal structure of CPAP-tubulin protein complex. To dissect the significance of this interaction, we generated transgenic Drosophilaand identified that this molecular interaction is critically required for centriole length determination (Zheng et al 2016 Nature Communications).We then extended our study and identified a chemical probe that prevents CPAP-tubulin interaction and stimulate centrosomes to recruit enhanced PCM (Mariappan et al 2019, The EMBO Journal).Finally, to understand the spatiotemporal regulation of PCM during cell cycle, we have identified that Sas-4 (in human it is CPAP) is being phosphorylated by Polo kinase, which is required to assemble PCM at the onset of mitosis (Ramani et al 2018 Cell).In this progress project report, we summarize our results. Collectively, our interdisciplinary collaborative studies have provided new and fundamental mechanistic insights into the assembly of a functional centrosome and centrosomal mechanisms those can be targeted in cancer cells.
Centrosomes are conserved organelles in eukaryotic cells consisting of centrioles surrounded by a protein network of peri-centriolar-material (PCM). Centrosomes are essential for fundamental cellular functions such as microtubule nucleation, accurate cell division and cilium formation. Within a centrosome, the centriole is the template for cilia formation during interphase, while the PCM assembles a spindle pole during mitosis to nucleate astral and spindle microtubules. In order for centrosomes to be functional organelle as a microtubule-organizing center (MTOC) of a cell, it must first successfully recruit PCM around centrioles. The complexity of PCM assembly, recruitment to centrosomes and their regulation remains a mystery.
By solving the co-crystal structure of PN2-3 domain of CPAP bound to tubulin dimer,we identified an unexpected role of CPAP PN2-3 in controlling centriolar and ciliary length. To uncover how centriolar and ciliary length is regulated, we undertook combinatorial approachthat
included biochemistry, structural biology and genetics. From the CPAP-tubulin crystal structure, we identified certain CPAP residues (CPAPEE343) to regulate the delivery of its bound cytoplasmic tubulin at the site of centriole and ciliary microtubule formation (Figure 1).
By generating human RPE1 cell linesand Drosophila expressing CPAPEE343RRand Sas-4EE112RR(fly counter part of CPAP) respectively, we identified that CPAP regulates delivery of its bound-tubulin to define the size of microtubule-based cellular structures of basal bodies and cilia (Zheng et al., 2016 Nature Commun).
Our studies in Drosophila have shown that cytoplasmic free tubulin negatively regulates the microtubule-nucleating activity of centrosomes through its direct interaction with Sas-4 (CPAP in humans) 18. Mutated Sas-4, which cannot interact with tubulin, prematurely activates interphase centrosomes to nucleate robust microtubules by recruiting increasing amounts of PCM proteins 18. To test this in cells, we established AlphaScreen, a proximity-based protein-protein interaction assay that identified CCB02, a selective inhibitor of CPAP-tubulin interaction. To do this, we exploited our recently solved CPAP-tubulin structure (Fig. 1). Further structural studies have indeed identified that CCB02 as a tubulin binder that competes for the CPAP binding site of β-tubulin, a previously uncharacterized site that has not been occupied by known conventional tubulin binders.
We use CBB02 not only to substantiate that tubulin negatively regulates CPAP-dependent peri-centriolar material recruitment but also to prematurely activate extra centrosomes of cancer cells. Here, we briefly explain our second year work, which is under review in Nature Chemical Biology.
Centrosome amplification is a hallmark of human cancers that can trigger cancer cell invasion.
To survive, cancer cells cluster extra centrosomes to achieve pseudo-bipolar division. Here, we set out to prevent clustering of extra centrosomes by prematurely activating centrosomes to induce microtubule nucleation before mitotic onset.
Tubulin, by interacting with the centrosomal protein CPAP, negatively regulates CPAP-dependent peri-centriolar material recruitment and concurrently microtubule nucleation. Screening for compounds that perturb CPAP-tubulin interaction lead to the identification of CCB02, which selectively binds at the CPAP binding site of tubulin.
CCB02 prematurely activates extra centrosomes to nucleate microtubules causing cancer cells to undergo centrosome declustering, multipolar mitosis, prolonged mitotic arrest and cell death (Fig. 2).
3D-organotypic invasive assays reveal CCB02 to have broad anti-invasive activity against various cancers including tyrosine-kinase inhibitor (TKI)-resistant EGFR- and KRAS-mutant non-small cell lung cancers. Thus, we identify a vulnerability of cancer cells to extra centrosomal activation, which may serve as a global approach to target various cancers including drug-resistant cancers.
1) Anand Ramani Mariappan A, Gottardo M, Mandad S, Urlaub H, Avidor-Reiss T, Riparbelli M, Callaini G, Debec A, Feederle R,et al., Gopalakrishnan J*.Plk1/Polo phosphorylates Sas-4 at the onset of mitosis for an efficient recruitment of pericentriolar material to centrosomes. Cell Reports. Dec 26;25(13):3618-3630.e6. doi: 10.1016/j.celrep.2018.11.102. (2018)
2) Arul Mariappan Soni K, Schorpp K, Zhao F, Minakar A, Zheng X, Mandad S, Macheleidt I, Ramani A, Kubelka T, Dawidowski M, Golfmann K, Wason A, Yang C, Simons J, Schmalz HG, Hyman AA, Aneja R, Ullrich R, Urlaub H, Odenthal M, Büttner R, Li H, Sattler M, Hadian K,Gopalakrishnan J*.Inhibition of CPAP-Tubulin interaction prevents the proliferation of centrosome amplified cancer cells.EMBO Journal Dec 10. pii: e99876. doi: 10.15252/embj.201899876(2018) (Highlighted in EMBO J as Magic Bullet).
3) Goranci-Buzhala G, Gabriel E, Mariappan A, Gopalakrishnan J*Losers of Primary Cilia Gain the Benefit of survival.Cancer Discovery 12, 1374-1375 (2017).
4) Zheng X, Ramani A, Soni K, Gottardo M, Zheng S, Ming Gooi L, Li W, Feng S, Mariappan A, Wason A, Widlund P, Pozniakovsky A, Poser I, Deng H, Ou G, Riparbelli M, Giuliano C, Hyman AA, Sattler M, Gopalakrishnan J*, Li H*. Molecular basis for CPAP-tubulin interaction in controlling centriolar and ciliary length.Nat Commun7, 11874 (2016).
5) Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, Kuivanen S, Korhonen E, Smura T, Vapalahti O, Papantonis A, Schmidt-Chanasit J, Riparbelli M, Callaini G, Krönke M, Utermöhlen O, Gopalakrishnan J*.Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids.Cell Stem Cell 20, 1-10 (2017).
Mariappan, A., Soni, K., Schorpp, K., Zhao, F., Minakar, A., Zheng, X., Mandad, S., Macheleidt, I., Ramani, A., Kubelka, T., Dawidowski, M., Golfmann, K., Wason, A., Yang, C., Simons, J., Schmalz, H.G., Hyman, A.A., Aneja, R., Ullrich, R., Urlaub, H., Odenthal, M., Buttner, R., Li, H., Sattler, M., Hadian, K., and Gopalakrishnan, J. (2019). Inhibition of CPAP-tubulin interaction prevents proliferation of centrosome-amplified cancer cells. EMBO J 38.
Mariappan A, Soni K, Schorpp K, Zhao F, Minakar A, Zheng X, Mandad S, Macheleidt I, Ramani A, Kubelka T, Dawidowski M, Golfmann K, Wason A, Yang C, Simons J, Schmalz HG, Hyman AA, Aneja R, Ullrich R, Urlaub H, Odenthal M, Buttner R, Li H, Sattler M, Hadian K, and Gopalakrishnan J (2018). Inhibition of CPAP-tubulin interaction prevents proliferation of centrosome-amplified cancer cells. EMBO J 10.15252/embj.201899876.
Martinez Carrera LA, Gabriel E, Donohoe CD, Holker I, Mariappan A, Storbeck M, Uhlirova M, Gopalakrishnan J, and Wirth B (2018). Novel insights into SMALED2: BICD2 mutations increase microtubule stability and cause defects in axonal and NMJ development. Hum Mol Genet 27, 1772-1784.
Ramani A, Mariappan A, Gottardo M, Mandad S, Urlaub H, Avidor-Reiss T, Riparbelli M, Callaini G, Debec A, Feederle R, and Gopalakrishnan J (2018). Plk1/Polo Phosphorylates Sas-4 at the Onset of Mitosis for an Efficient Recruitment of Pericentriolar Material to Centrosomes. Cell Rep 25, 3618-3630 e3616.
Gabriel E, and Gopalakrishnan J (2017). Generation of iPSC-derived Human Brain Organoids to Model Early Neurodevelopmental Disorders. J Vis Exp 10.3791/55372.
Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, Kuivanen S, Korhonen E, Smura T, Vapalahti O, Papantonis A, Schmidt-Chanasit J, Riparbelli M, Callaini G, Kronke M, Utermohlen O, and Gopalakrishnan J (2017). Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 20, 397-406 e5.
Goranci-Buzhala G, Gabriel E, Mariappan A, and Gopalakrishnan J (2017). Losers of Primary Cilia Gain the Benefit of Survival. Cancer Discov 7, 1374-5.
Brenke JK, Salmina ES, Ringelstetter L, Dornauer S, Kuzikov M, Rothenaigner I, Schorpp K, Giehler F, Gopalakrishnan J, Kieser A, Gul S, Tetko IV, and Hadian K (2016). Identification of small-molecule frequent hitters of glutathione s-transferase-glutathione interaction. J Biomol Screen 10.1177/1087057116639992.
Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F, Saric T, Hescheler J, Hyman AA, Gottardo M, Callaini G, Alkuraya FS, and Gopalakrishnan J (2016). Cpap promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J 35, 803-819.
Thomopoulou P, Sachs J, Teusch N, Mariappan A, Gopalakrishnan J, and Schmalz HG (2016). New colchicine-derived triazoles and their influence on cytotoxicity and microtubule morphology. ACS Med Chem Lett 7, 188-191.
Wike CL, Graves HK, Wason A, Hawkins R, Gopalakrishnan J, Schumacher J, and Tyler JK (2016). Excess free histone h3 localizes to centrosomes for proteasome-mediated degradation during mitosis in metazoans. Cell Cycle 10.1080/15384101.2016.1192728, 1-10.
Zheng X, Ramani A, Soni K, Gottardo M, Zheng S, Ming Gooi L, Li W, Feng S, Mariappan A, Wason A, Widlund P, Pozniakovsky A, Poser I, Deng H, Ou G, Riparbelli M, Giuliano C, Hyman AA, Sattler M, Gopalakrishnan J, and Li H (2016). Molecular basis for cpap-tubulin interaction in controlling centriolar and ciliary length. Nat Commun 7, 11874.
Avidor-Reiss T, and Gopalakrishnan J (2013). Building a centriole. Curr Opin Cell Biol 25, 72-77.
Avidor-Reiss T, and Gopalakrishnan J (2013). Cell cycle Regulation of the centrosome and cilium. Drug Discov Today Dis Mech 10, e119-e124
Pannu V, Rida PC, Celik B, Turaga RC, Ogden A, Cantuaria G, Gopalakrishnan J, and Aneja R (2014). Centrosome-declustering drugs mediate a two-pronged attack on interphase and mitosis in supercentrosomal cancer cells. Cell death & disease 5, e1538.
Schorpp K, Rothenaigner I, Salmina E, Reinshagen J, Low T, Brenke JK, Gopalakrishnan J, Tetko IV, Gul S, and Hadian K (2014). Identification of small-molecule frequent hitters from alphascreen high-throughput screens. J Biomol Screen 19, 715-726.
Zheng X, Gooi LM, Wason A, Gabriel E, Mehrjardi NZ, Yang Q, Zhang X, Debec A, Basiri ML, Avidor-Reiss T, Pozniakovsky A, Poser I, Saric T, Hyman AA, Li H, and Gopalakrishnan J (2014). Conserved tcp domain of sas-4/cpap is essential for pericentriolar material tethering during centrosome biogenesis. Proc Natl Acad Sci U S A 111, E354-363.
Information from this funding period will not be updated anymore. New research related information is available here.

CMMC Affiliation
assoc.RG (since 08/2018) Principal Investigator - CMMC-JRG IX (11/2012-07/2018)
jay.gopalakrishnan[at]uni-koeln.de
show more…+49 221 478 89691
+49 221 478 5949
CMMC Affiliation
present address: Universitätsstr. 1
40225 Düsseldorf
http://centrosome-cilia-lab.com/
Curriculum Vitae (CV)
Dr. Elke Gabriel (PostDoc)
Arul Mariappan (PostDoc)
Dr. Anand Ramani (PostDoc)
Gladiola-Goranci Buzchala (Doctoral student)
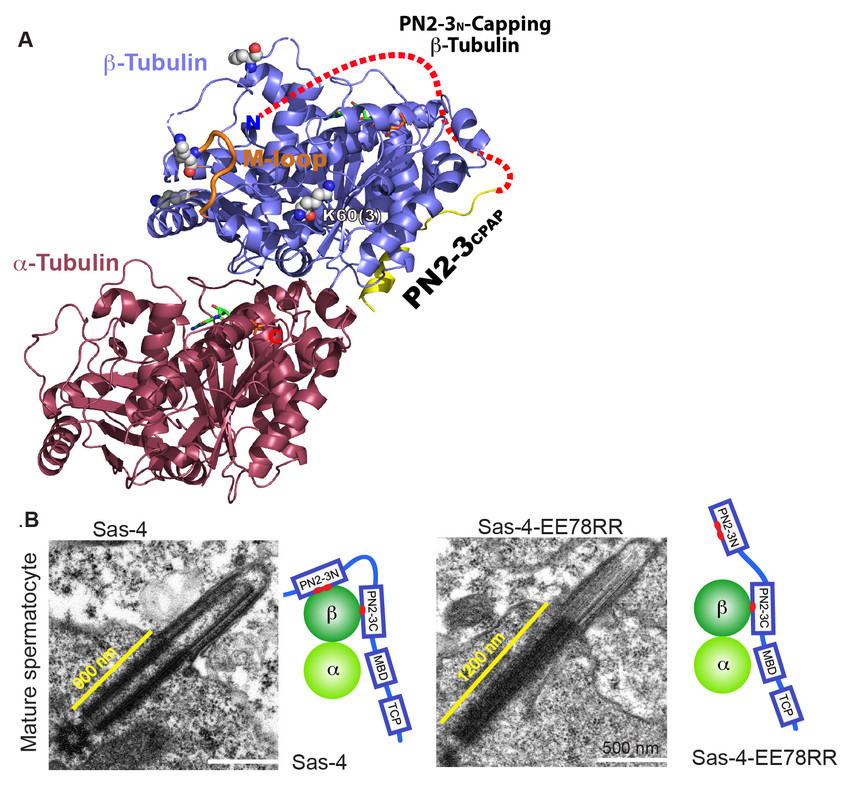
Figure1.(A)A 2.2Å crystal structure of conserved PN2-3 domain of CPAP in complex with tubulin hetero- dimer. While the C-terminal PN2-3 binds the β-tubulin’s outer surface, the N-terminal PN2-3 caps microtubule’s α-βsurface of β-tubulin (Scheme).
(B)EM micrographs of Drosophilaspermatocyte cells. While the wild type protein controls the basal body and primary cilium size, the mutant Sas-4EE112RR which unmasks the β-tubulin surface required for polymerization displays an elongated basal body and cilium.
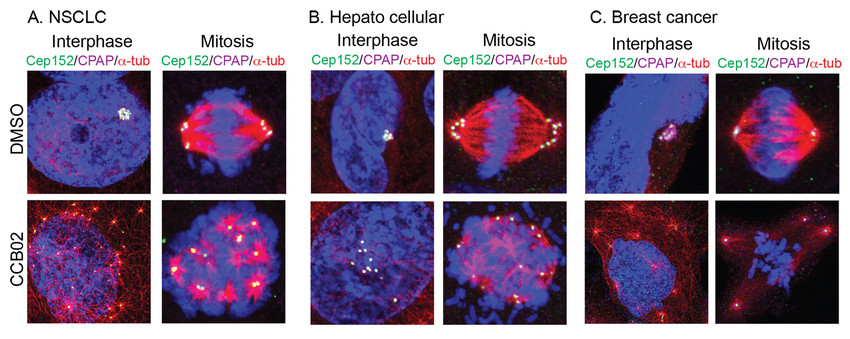
Figure 2.Effects of CCB02 on EGFR mutant NSCLC (A),hepatocellular (B)and breast cancer cells (C).DMSO control shows clustered centrosomes. Compound treatment effectively de-clustered both interphase and mitotic centrosomes at concentrations ranging from 0.5 to 5 M. Only representative images are shown.