The focus of our research is to provide holistic genetic, biochemical and cell biological insight into the systemic physiological role of a) ω3- and ω6-polyunsaturated fatty acids (PUFAs) and b) sphingolipids as essential structures in the complex architecture of cellular membranes and as precursor of a wide variety of lipophilic signalling and their molecular role in pathogenetic mechanisms of PUFA-deficiency related diseases and growth inhibition in dwarfism and chondrodysplasia.
In our first research-project the fads2null mouse (PUFA-deficient) mutant has provided extensive insight into the role of ω3-and ω6-poly-unsaturated fatty acids in the regulation of the systemic lipid metabolism and in the development of metabolic diseases, and phospholipids as constituents of the architecture of lipid bilayers of membranes in CNS and extra- neuronal tissues.
In our second project we provided insight into the mechanism underlying the novel function of neutral sphingomyelinase and sphingolipids in a basic cellular process, the canonic Golgi secretory protein pathway and in the smpd3null mutant in its molecular pathology.
Δ-6-fatty acid desaturase (FADS2) is the key enzyme in the biosynthesis of polyunsaturated fatty acids (PUFAs), the essential structural determinants of mammalian membrane lipid-bilayers. We developed the auxotrophic fads2-/- mouse mutant to assess the enigmatic role of ω- 3- and ω-6-PUFAs in lipid homeostasis, membrane structure and function.
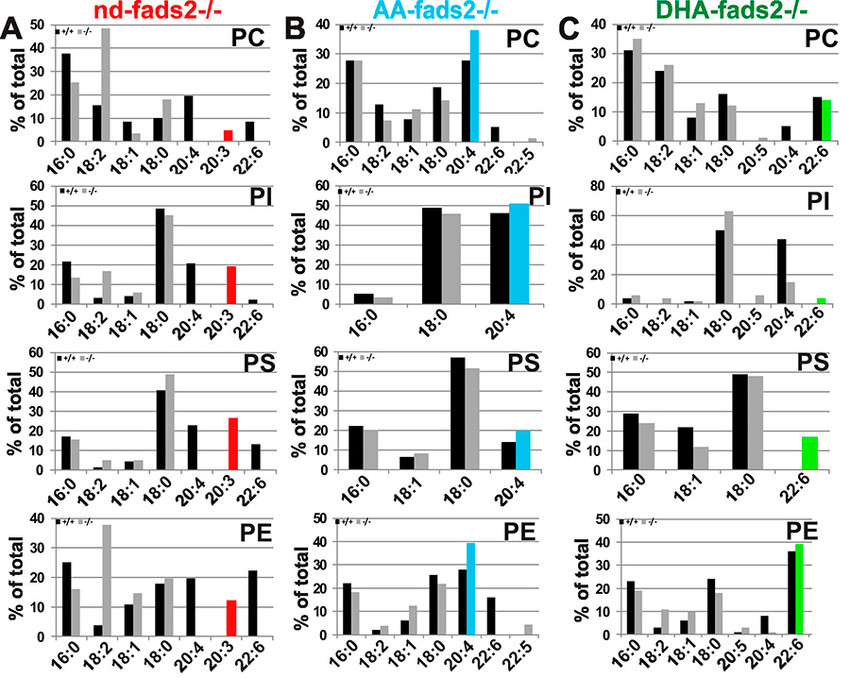
Obesity resistance is another major phenotype of the fads2-/- mutant, the molecular basis of which is unknown. Phospholipidomic profiling of membrane systems of fads2-/- mice revealed diacylglycerol structures, deprived of PUFAs but substituted with surrogate eicosa-5,11,14- trienoic acid. ω6-Arachidonic (AA) and ω3-docosahexaenoic acid (DHA) supplemented diets transformed fads2-/- into AA-fads2-/- and DHA-fads2-/- mutants.
Severely altered phospholipid-bilayer structures of subcellular membranes of fads2-/- liver specifically interfered with maturation of transcription factor sterol-regulatory- element-binding protein (SREBP1c), the key regulator of lipogenesis and lipid homeostasis. This study strengthens the concept that specific PUFA-substituted membrane phospholipid species are critical constituents of the structural platform, operative in lipid homeostasis in normal and disease conditions1.
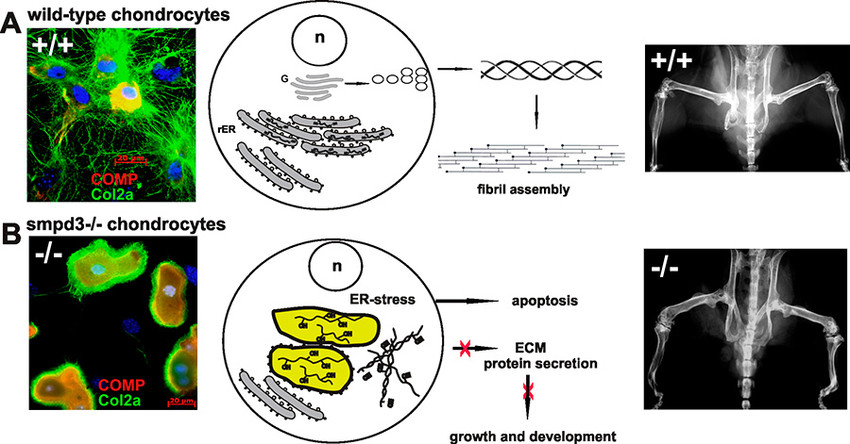
In our second project, we have substantiated the pivotal function of the brain specific SMPD3 in a novel essential membrane lipid bilayer modelling reaction, essential in Golgi vesicular protein transport in the smpd3-/- mouse model.
Studies in smpd3-/- mice and SMPD3-deficient primary chondrocytes unveiled the molecular basis underlying the dichotomic smpd3-dwarf phenotype. SMPD3-deficiency disrupted the Golgi SMPD3/SMS1 cycle, which regulates lipid driven membrane remodeling in bud and vesicle formation. SMPD3 deficiency caused disruption of the Golgi secretory pathway in growth promoting tissues, dysproteostasis, ER- stress and premature apoptosis, manifested in the skeletal growth inhibition, malformation and chondro-dysplasia in this novel smpd3-dwarf phenotype2.
The genetically and biochemically well-defined auxotrophic fads2-/- and the derived AA- and DHA fads2-/- mutant mouse lines preclude many of the ambiguities of numerous feeding experiments in rodents in the past, which addressed the important role of PUFAs. This study indicates the wide experimental scope of the auxotrophic fads2-/- mouse. The nd-, AA- and DHA-/-mouse lines have proven useful models for unveiling complex mechanisms underlying the molecular function and imbalanced dietary PUFA supply of dietary ω3- and ω6-PUFAs in membrane biology, human nutrition and the development and prevention of dyslipidemia, vascular and neurodegenerative diseases.
This unprecedented aspect of SMPD3 function necessitates a reconsideration of the current tenet regarding the role of Smases and sphingomyelin metabolites in intracellular signal transduction pathways. SMPD3 is most abundantly expressed in brain. This study suggests further exploration of the function of SMPD3 in the Golgi vesicular intracellular protein transport and secretion in neurons of the cerebral cortex and hippocampus. Expansion of this concept to other rapidly growing cells, e.g. in tumors, the immune system, and inflammation awaits further studies and might open new perspectives for therapeutic strategies.
Stoffel, W., Hammels, I. , Jenke, B., Binczek, E., Schmidt-Soltau, I., Brodesser, S., Odenthal, M. and Thevis, M. (2014) Obesity resistance and deregulation of lipogenesis in Δ6-fatty acid desaturase (FADS2) deficiency. EMBOR 15, 110-120.
Stoffel, W., Hammels, I. , Jenke, B., Binczek, E., Schmidt-Soltau, I., Brodesser, S., Schauss, A., Heilig, J., Zauke, F. (2016) Neutral sphingomyelinase (SMPD3) deficiency disrupts the Golgi secretory pathway and causes growth inhibition. Cell Death & Disease accepted for publication.
Information from this funding period will not be updated anymore. New research related information is available here.

Laboratory of Molecular Neuroscience - Center for Biochemistry
CMMC - PI - SRG 01
Executive Board Member
wilhelm.stoffel[at]uni-koeln.de
show more…+49 221 478 6881
+49 221 478 6882
Laboratory of Molecular Neuroscience - Center for Biochemistry
Joseph-Stelzmann-Str. 52
50931 Cologne
Dr. Ina Hammels (PostDoc)
Britta Jenke (technician)
Inga Schmidt-Soltau (technician)